Secondary amyloidosis pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 14: | Line 14: | ||
== Associated Conditions == | == Associated Conditions == | ||
*[[Conditions]] associated with AA amyloidosis include:<ref name="BlankHegenbart2018">{{cite journal|last1=Blank|first1=Norbert|last2=Hegenbart|first2=Ute|last3=Dietrich|first3=Sascha|last4=Brune|first4=Maik|last5=Beimler|first5=Jörg|last6=Röcken|first6=Christoph|last7=Müller-Tidow|first7=Carsten|last8=Lorenz|first8=Hanns-Martin|last9=Schönland|first9=Stefan O.|title=Obesity is a significant susceptibility factor for idiopathic AA amyloidosis|journal=Amyloid|volume=25|issue=1|year=2018|pages=37–45|issn=1350-6129|doi=10.1080/13506129.2018.1429391}}</ref><ref name="van der HilstYamada2008">{{cite journal|last1=van der Hilst|first1=J. C. H.|last2=Yamada|first2=T.|last3=Op den Camp|first3=H. J. M.|last4=van der Meer|first4=J. W. M.|last5=Drenth|first5=J. P. H.|last6=Simon|first6=A.|title=Increased susceptibility of serum amyloid A 1.1 to degradation by MMP-1: potential explanation for higher risk of type AA amyloidosis|journal=Rheumatology|volume=47|issue=11|year=2008|pages=1651–1654|issn=1462-0324|doi=10.1093/rheumatology/ken371}}</ref><ref name="PapaDoglio2017">{{cite journal|last1=Papa|first1=Riccardo|last2=Doglio|first2=Matteo|last3=Lachmann|first3=Helen J.|last4=Ozen|first4=Seza|last5=Frenkel|first5=Joost|last6=Simon|first6=Anna|last7=Neven|first7=Bénédicte|last8=Kuemmerle-Deschner|first8=Jasmin|last9=Ozgodan|first9=Huri|last10=Caorsi|first10=Roberta|last11=Federici|first11=Silvia|last12=Finetti|first12=Martina|last13=Trachana|first13=Maria|last14=Brunner|first14=Jurgen|last15=Bezrodnik|first15=Liliana|last16=Pinedo Gago|first16=Mari Carmen|last17=Maggio|first17=Maria Cristina|last18=Tsitsami|first18=Elena|last19=Al Suwairi|first19=Wafaa|last20=Espada|first20=Graciela|last21=Shcherbina|first21=Anna|last22=Aksu|first22=Guzide|last23=Ruperto|first23=Nicolino|last24=Martini|first24=Alberto|last25=Ceccherini|first25=Isabella|last26=Gattorno|first26=Marco|title=A web-based collection of genotype-phenotype associations in hereditary recurrent fevers from the Eurofever registry|journal=Orphanet Journal of Rare Diseases|volume=12|issue=1|year=2017|issn=1750-1172|doi=10.1186/s13023-017-0720-3}}</ref> | *[[Conditions]] associated with AA amyloidosis include:<ref name="BlankHegenbart2018">{{cite journal|last1=Blank|first1=Norbert|last2=Hegenbart|first2=Ute|last3=Dietrich|first3=Sascha|last4=Brune|first4=Maik|last5=Beimler|first5=Jörg|last6=Röcken|first6=Christoph|last7=Müller-Tidow|first7=Carsten|last8=Lorenz|first8=Hanns-Martin|last9=Schönland|first9=Stefan O.|title=Obesity is a significant susceptibility factor for idiopathic AA amyloidosis|journal=Amyloid|volume=25|issue=1|year=2018|pages=37–45|issn=1350-6129|doi=10.1080/13506129.2018.1429391}}</ref><ref name="van der HilstYamada2008">{{cite journal|last1=van der Hilst|first1=J. C. H.|last2=Yamada|first2=T.|last3=Op den Camp|first3=H. J. M.|last4=van der Meer|first4=J. W. M.|last5=Drenth|first5=J. P. H.|last6=Simon|first6=A.|title=Increased susceptibility of serum amyloid A 1.1 to degradation by MMP-1: potential explanation for higher risk of type AA amyloidosis|journal=Rheumatology|volume=47|issue=11|year=2008|pages=1651–1654|issn=1462-0324|doi=10.1093/rheumatology/ken371}}</ref><ref name="PapaDoglio2017">{{cite journal|last1=Papa|first1=Riccardo|last2=Doglio|first2=Matteo|last3=Lachmann|first3=Helen J.|last4=Ozen|first4=Seza|last5=Frenkel|first5=Joost|last6=Simon|first6=Anna|last7=Neven|first7=Bénédicte|last8=Kuemmerle-Deschner|first8=Jasmin|last9=Ozgodan|first9=Huri|last10=Caorsi|first10=Roberta|last11=Federici|first11=Silvia|last12=Finetti|first12=Martina|last13=Trachana|first13=Maria|last14=Brunner|first14=Jurgen|last15=Bezrodnik|first15=Liliana|last16=Pinedo Gago|first16=Mari Carmen|last17=Maggio|first17=Maria Cristina|last18=Tsitsami|first18=Elena|last19=Al Suwairi|first19=Wafaa|last20=Espada|first20=Graciela|last21=Shcherbina|first21=Anna|last22=Aksu|first22=Guzide|last23=Ruperto|first23=Nicolino|last24=Martini|first24=Alberto|last25=Ceccherini|first25=Isabella|last26=Gattorno|first26=Marco|title=A web-based collection of genotype-phenotype associations in hereditary recurrent fevers from the Eurofever registry|journal=Orphanet Journal of Rare Diseases|volume=12|issue=1|year=2017|issn=1750-1172|doi=10.1186/s13023-017-0720-3}}</ref> | ||
** | {| class="wikitable" | ||
***[[ | |+ | ||
***[[TNF receptor associated periodic syndrome|TNF Receptor associated periodic syndrome]] ([[TRAPS]]) | | colspan="2" style="background:#4479BA; color: #FFFFFF;" align="center" + |'''Associated Conditions''' | ||
|- | |||
| style="background: #DCDCDC; padding: 5px; text-align: left;" |'''Conditions''' | |||
|'''Examples''' | |||
|- | |||
| style="background: #DCDCDC; padding: 5px; text-align: left;" |'''Chronic infections''' | |||
| | |||
*[[Tuberculosis]] | |||
*[[Leprosy]] | |||
*Whipple Disease | |||
*[[Osteomyelitis]] | |||
*[[Chronic pyelonephritis]] | |||
*[[Subacute bacterial endocarditis]] | |||
*Chronic cutaneous ulcers | |||
|- | |||
| style="background: #DCDCDC; padding: 5px; text-align: left;" |'''Monogenic [[Periodic fever syndrome|periodic fever syndromes]]''' | |||
|<br /> | |||
*[[TNF receptor associated periodic syndrome|TNF Receptor associated periodic syndrome]] ([[TRAPS]]) | |||
*[[CAPS|Cryopyrin-associated periodic fever syndrome]] | |||
*[[Mevalonate kinase deficiency]] | |||
*[[Familial mediterranean fever]] | |||
|- | |||
| style="background: #DCDCDC; padding: 5px; text-align: left;" |'''Conditions predisposing to recurrent infections''' | |||
| | |||
*[[Cystic fibrosis]] | |||
*[[Bronchiectasis]] | |||
*[[Kartagener syndrome]] | |||
*[[Epidermolysis bullosa]] | |||
*[[IV drug abusers|Injected drug abuse]] | |||
*[[Jejuno-ileal bypass]] | |||
*[[Paraplegia]] | |||
*[[Sickle cell anemia]] | |||
*[[Immunodeficiency]] | |||
*[[Common variable immunodeficiency]] | |||
*[[Cyclic neutropenia]] | |||
*[[Hyperimmunoglobulin M syndrome]] | |||
*[[Hypogammaglobulinemia]] | |||
*[[X-linked agammaglobulinemia|Sex-linked agammaglobulinemia]] | |||
*[[Human Immunodeficiency Virus|Human immunodeficiency virus]]/[[AIDS]] | |||
|- | |||
| style="background: #DCDCDC; padding: 5px; text-align: left;" |'''Neoplasia''' | |||
| | |||
*[[Adenocarcinoma]] | |||
*[[Basal cell carcinoma]] | |||
*[[Carcinoid tumors|Carcinoid tumor]] | |||
*[[Castleman disease]] | |||
*[[Gastrointestinal stromal tumor]] | |||
*[[Hairy cell leukemia]] | |||
*[[Hepatic adenoma]] | |||
*[[Hodgkin disease]] | |||
*[[Mesothelioma]] | |||
*[[Renal cell carcinoma]] | |||
*[[Sarcoma]] | |||
|- | |||
| style="background: #DCDCDC; padding: 5px; text-align: left;" |'''Inflammatory Arthritis''' | |||
| | |||
*Adult-onset Still disease | |||
*[[Ankylosing spondylitis]] | |||
*[[Juvenile idiopathic arthritis]] | |||
*[[Psoriatic arthropathy]] | |||
*[[Reiter's syndrome|Reiter syndrome]] | |||
*[[Rheumatoid arthritis]] | |||
*[[Gout]] | |||
|- | |||
| style="background: #DCDCDC; padding: 5px; text-align: left;" |'''Systemic [[Vasculitis]]''' | |||
| | |||
*Antineutrophil cytoplasmic antibody-associated vasculitis | |||
*[[Behcet disease]] | |||
*[[Giant cell arteritis]] | |||
*[[Polyarteritis nodosa]] | |||
*[[Polymyalgia rheumatica]] | |||
*[[Systemic lupus erythematosus]] | |||
*[[Takayasu arteritis]] | |||
*[[Inflammatory Bowel Disease]] | |||
*[[Ulcerative colitis]] | |||
*[[Crohn disease]] | |||
|- | |||
| style="background: #DCDCDC; padding: 5px; text-align: left;" |'''Others''' | |||
| | |||
*[[Atrial myxoma]] | |||
*Inflammatory abdominal aortic aneurism | |||
*[[Retroperitoneal fibrosis]] | |||
*[[SAPHO syndrome|SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis) syndrome]] | |||
*[[Sarcoidosis]] | |||
*[[Sinus histiocytosis with massive lymphadenopathy]] | |||
|- | |||
|+ | |||
|- | |||
|+ | |||
|- | |||
|- | |||
|} | |||
== Gross Pathology == | == Gross Pathology == | ||
On [[gross pathology]], the organs affected by amyloidosis can be characterized by the following features: | On [[gross pathology]], the organs affected by amyloidosis can be characterized by the following features: | ||
Revision as of 21:20, 7 November 2019
|
Secondary amyloidosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Secondary amyloidosis pathophysiology On the Web |
|
American Roentgen Ray Society Images of Secondary amyloidosis pathophysiology |
|
Risk calculators and risk factors for Secondary amyloidosis pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sahar Memar Montazerin, M.D.[2]Shaghayegh Habibi, M.D. Sabawoon Mirwais, M.B.B.S, M.D.
Overview
AA amyloid is an abnormal insoluble extracellular protein derived from the spontaneous aggregation of fibrils composed of misfolded proteins, called amyloid precursors. In secondary amyloidosis, the amyloid precursor is SAA, an acute phase reactant, encoded by SAA 1&2 genes. In secondary amyloidosis, intermediate products of SAA metabolism tend to aggregate and form protofilaments that accumulate within extracellular space and form the insoluble amyloid deposits. Secondary amyloidosis is associated with chronic inflammation (such as tuberculosis or rheumatoid arthritis).
Pathophysiology
- AA amyloid is an abnormal insoluble extracellular protein derived from the spontaneous aggregation of fibrils composed of misfolded proteins, called amyloid precursors.[1][2]
- In secondary amyloidosis, the amyloid precursor is SAA, an acute phase reactant, encoded by SAA 1&2 genes.
- Normally, SAA increased during the inflammatory process and then taken up by macrophages and degraded within lysosomal compartments.
- In patients with secondary amyloidosis, intermediate products of SAA metabolism tend to aggregate and form protofilaments.
- These protofilaments with other protein materials accumulate within extracellular space and form the insoluble amyloid deposits.
- Amyloid deposition can disrupt tissue structure of involved organ and consequently leads to organ failure.[3]
Associated Conditions
- Conditions associated with AA amyloidosis include:[4][5][6]
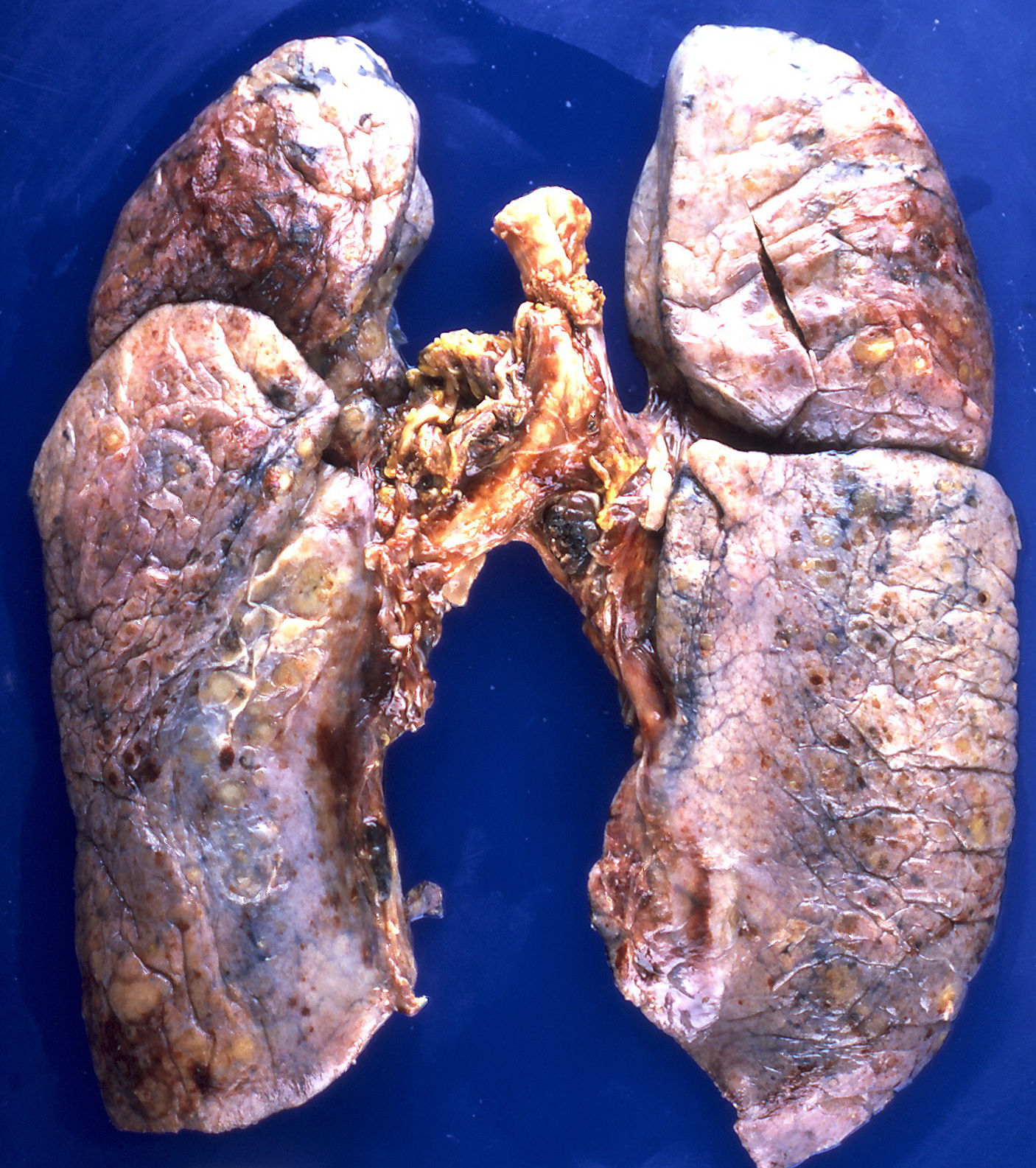
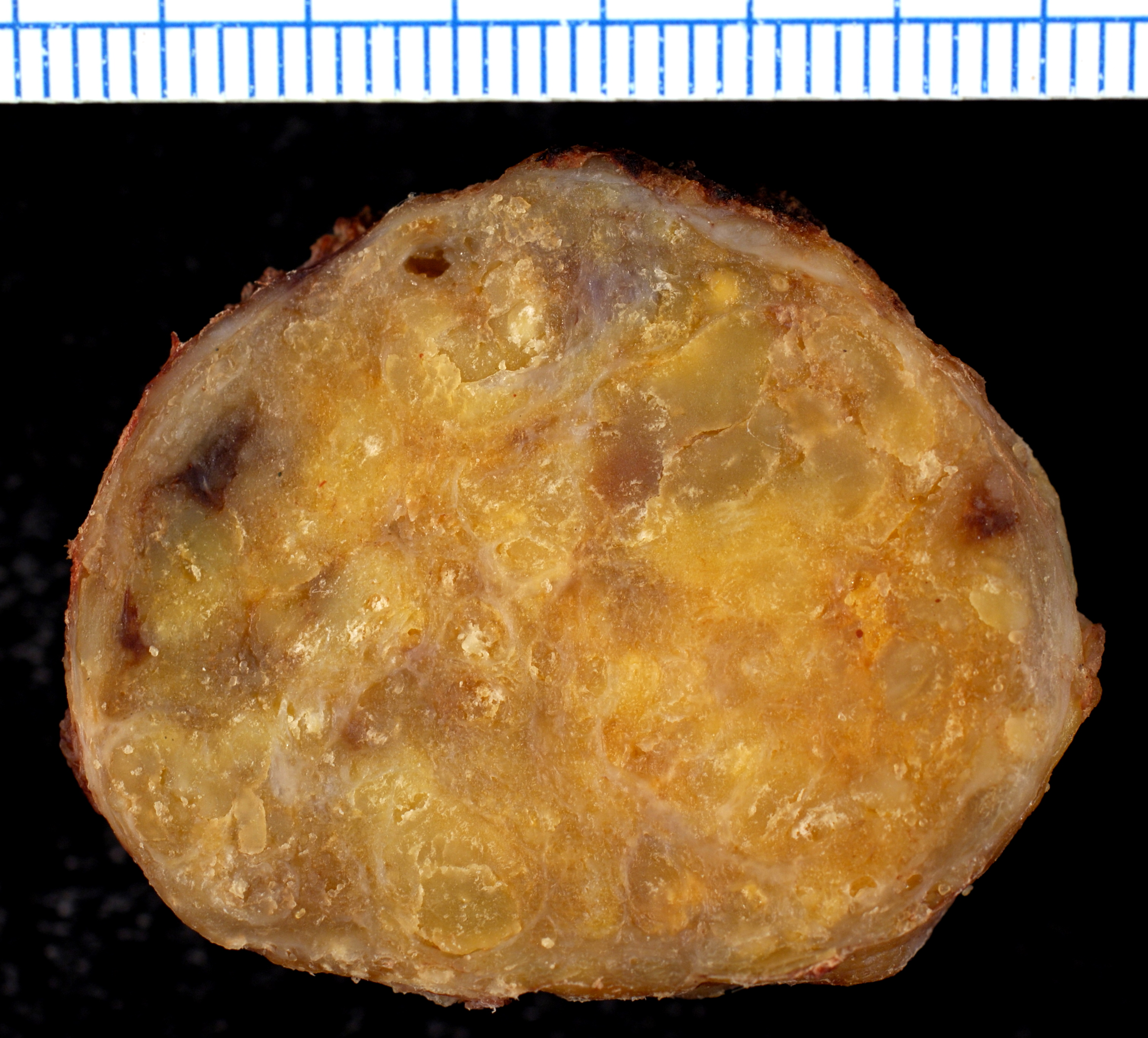
Gross Pathology
On gross pathology, the organs affected by amyloidosis can be characterized by the following features:
- Porcelain like or waxy appearance
- Enlargement
Images

Microscopic Pathology
On microscopic histopathological analysis, aa amyloidosis is characterized by:[10][11]
- Green birefringence under polarized light after Congo red staining (appears red under normal light)
- Linear non-branching fibrils (indefinite length with an approximately same diameter)
- Distinct X-ray diffraction pattern consistent with Pauling's model of a cross-beta fibril
On electron microscopy, amyloid fibrils have the following characteristics:[12]
- 10 to 15 nm diameter
- Straight, rigid, and nonbranching
- Composed of twisted protofilaments
Images



References
- ↑ Jayaraman, Shobini; Gantz, Donald L.; Haupt, Christian; Gursky, Olga (2017). "Serum amyloid A forms stable oligomers that disrupt vesicles at lysosomal pH and contribute to the pathogenesis of reactive amyloidosis". Proceedings of the National Academy of Sciences. 114 (32): E6507–E6515. doi:10.1073/pnas.1707120114. ISSN 0027-8424.
- ↑ Kisilevsky, Robert; Raimondi, Sara; Bellotti, Vittorio (2016). "Historical and Current Concepts of Fibrillogenesis and In vivo Amyloidogenesis: Implications of Amyloid Tissue Targeting". Frontiers in Molecular Biosciences. 3. doi:10.3389/fmolb.2016.00017. ISSN 2296-889X.
- ↑ Wechalekar AD, Gillmore JD, Hawkins PN (June 2016). "Systemic amyloidosis". Lancet. 387 (10038): 2641–2654. doi:10.1016/S0140-6736(15)01274-X. PMID 26719234.
- ↑ Blank, Norbert; Hegenbart, Ute; Dietrich, Sascha; Brune, Maik; Beimler, Jörg; Röcken, Christoph; Müller-Tidow, Carsten; Lorenz, Hanns-Martin; Schönland, Stefan O. (2018). "Obesity is a significant susceptibility factor for idiopathic AA amyloidosis". Amyloid. 25 (1): 37–45. doi:10.1080/13506129.2018.1429391. ISSN 1350-6129.
- ↑ van der Hilst, J. C. H.; Yamada, T.; Op den Camp, H. J. M.; van der Meer, J. W. M.; Drenth, J. P. H.; Simon, A. (2008). "Increased susceptibility of serum amyloid A 1.1 to degradation by MMP-1: potential explanation for higher risk of type AA amyloidosis". Rheumatology. 47 (11): 1651–1654. doi:10.1093/rheumatology/ken371. ISSN 1462-0324.
- ↑ Papa, Riccardo; Doglio, Matteo; Lachmann, Helen J.; Ozen, Seza; Frenkel, Joost; Simon, Anna; Neven, Bénédicte; Kuemmerle-Deschner, Jasmin; Ozgodan, Huri; Caorsi, Roberta; Federici, Silvia; Finetti, Martina; Trachana, Maria; Brunner, Jurgen; Bezrodnik, Liliana; Pinedo Gago, Mari Carmen; Maggio, Maria Cristina; Tsitsami, Elena; Al Suwairi, Wafaa; Espada, Graciela; Shcherbina, Anna; Aksu, Guzide; Ruperto, Nicolino; Martini, Alberto; Ceccherini, Isabella; Gattorno, Marco (2017). "A web-based collection of genotype-phenotype associations in hereditary recurrent fevers from the Eurofever registry". Orphanet Journal of Rare Diseases. 12 (1). doi:10.1186/s13023-017-0720-3. ISSN 1750-1172.
- ↑ By Yale Rosen from USA - Amyloidosis, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=31127928
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377537238/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629764
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377538012/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629740
- ↑
- ↑ Röcken C, Shakespeare A (February 2002). "Pathology, diagnosis and pathogenesis of AA amyloidosis". Virchows Arch. 440 (2): 111–122. doi:10.1007/s00428-001-0582-9. PMID 11964039.
- ↑ Close, William; Neumann, Matthias; Schmidt, Andreas; Hora, Manuel; Annamalai, Karthikeyan; Schmidt, Matthias; Reif, Bernd; Schmidt, Volker; Grigorieff, Nikolaus; Fändrich, Marcus (2018). "Physical basis of amyloid fibril polymorphism". Nature Communications. 9 (1). doi:10.1038/s41467-018-03164-5. ISSN 2041-1723.
- ↑ By Michael Feldman, MD, PhDUniversity of Pennsylvania School of Medicine - http://www.healcentral.org/healapp/showMetadata?metadataId=38717, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=870218
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377559787/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629716
- ↑ By Ed Uthman, MD - https://www.flickr.com/photos/euthman/377559955/, CC BY-SA 2.0, https://commons.wikimedia.org/w/index.php?curid=1629705