Myeloproliferative neoplasm pathophysiology: Difference between revisions
Gerald Chi (talk | contribs) mNo edit summary |
Gerald Chi (talk | contribs) m (→Genetics) |
||
| Line 12: | Line 12: | ||
===Genetics=== | ===Genetics=== | ||
Primary cytogenetic abnormalities have not been identified in the majority of myeloproliferative neoplasms. Aberrant activation of [[tyrosine kinase]]s and associated signaling pathways is frequently implicated as the disease-initiating event. | |||
====Chronic Myelogenous Leukemia==== | ====Chronic Myelogenous Leukemia==== | ||
{{Main|Chronic myelogenous leukemia pathophysiology}} | {{Main|Chronic myelogenous leukemia pathophysiology}} | ||
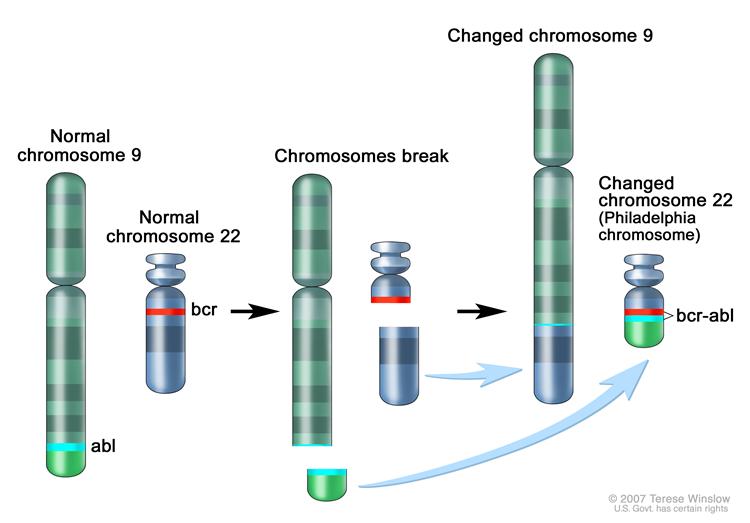
In [[chronic myelogenous leukemia]], | In [[chronic myelogenous leukemia]], a balanced reciprocal [[translocation]] termed t(9;22)(q34;q11.2) results in formation of the [[BCR]]-[[ABL]] hybrid gene which encodes for the p210<sup>BCR-ABL</sup> oncoprotein. Constitutive activation of the [[BCR]]-[[ABL]] [[oncogene]] and downstream signaling pathways confers survival advantage to leukemic cells and suppresses normal [[hematopoiesis]]. | ||
====Polycythemia Vera==== | ====Polycythemia Vera==== | ||
{{Main|Polycythemia vera pathophysiology}} | {{Main|Polycythemia vera pathophysiology}} | ||
In [[polycythemia vera]], | In [[polycythemia vera]], [[erythroid]] [[progenitors]] demonstrate hypersensitivity to [[insulin-like growth factor-1|insulin-like growth factor-1 (IGF-1)]] and other [[cytokines]]. Mutations in the [[Janus kinase 2]] gene, particularly JAK2V617V, may contribute to [[cytokine]]-independent proliferation of [[CD34]]+ [[hematopoietic]] [[progenitors]] and their progeny. | ||
====Primary Myelofibrosis==== | ====Primary Myelofibrosis==== | ||
{{Main|Myelofibrosis pathophysiology}} | {{Main|Myelofibrosis pathophysiology}} | ||
Primary myelofibrosis | Primary myelofibrosis is hallmarked by clonal myeloproliferation with reactive stromal changes in response to an uncontrolled production of growth factors (e.g., [[TGF beta|transforming growth factor β]], [[platelet-derived growth factor|platelet-derived growth factor]], and [[basic fibroblast growth factor|basic fibroblast growth factor]]) from resident megakaryocyes and monocytes. Etiopathogenic mutations leading to primary myelofibrosis remain unclear.<ref>{{cite book | last = Jaffe | first = Elaine | title = Pathology and genetics of tumours of haematopoietic and lymphoid tissues | publisher = IARC Press Oxford University Press | year = 2001 | isbn = 978-9283224112 }}</ref> | ||
==References== | ==References== | ||
Revision as of 21:47, 1 April 2015
|
Myeloproliferative Neoplasm Microchapters |
|
Differentiating myeloproliferative neoplasm from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Myeloproliferative neoplasm pathophysiology On the Web |
|
American Roentgen Ray Society Images of Myeloproliferative neoplasm pathophysiology |
|
Directions to Hospitals Treating Myeloproliferative neoplasm |
|
Risk calculators and risk factors for Myeloproliferative neoplasm pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Clinical and pathologic findings in the myeloproliferative neoplasms (MPNs) are due to dysregulated proliferation and expansion of myeloid progenitors in the bone marrow, resulting in altered populations of granulocytes, erythrocytes, or platelets in the peripheral blood.
Pathophysiology
 |
Genetics
Primary cytogenetic abnormalities have not been identified in the majority of myeloproliferative neoplasms. Aberrant activation of tyrosine kinases and associated signaling pathways is frequently implicated as the disease-initiating event.
Chronic Myelogenous Leukemia
In chronic myelogenous leukemia, a balanced reciprocal translocation termed t(9;22)(q34;q11.2) results in formation of the BCR-ABL hybrid gene which encodes for the p210BCR-ABL oncoprotein. Constitutive activation of the BCR-ABL oncogene and downstream signaling pathways confers survival advantage to leukemic cells and suppresses normal hematopoiesis.
Polycythemia Vera
In polycythemia vera, erythroid progenitors demonstrate hypersensitivity to insulin-like growth factor-1 (IGF-1) and other cytokines. Mutations in the Janus kinase 2 gene, particularly JAK2V617V, may contribute to cytokine-independent proliferation of CD34+ hematopoietic progenitors and their progeny.
Primary Myelofibrosis
Primary myelofibrosis is hallmarked by clonal myeloproliferation with reactive stromal changes in response to an uncontrolled production of growth factors (e.g., transforming growth factor β, platelet-derived growth factor, and basic fibroblast growth factor) from resident megakaryocyes and monocytes. Etiopathogenic mutations leading to primary myelofibrosis remain unclear.[2]
References
- ↑ "Chronic Myelogenous Leukemia Treatment".
- ↑ Jaffe, Elaine (2001). Pathology and genetics of tumours of haematopoietic and lymphoid tissues. IARC Press Oxford University Press. ISBN 978-9283224112.