Terbinafine (topical): Difference between revisions
Gerald Chi (talk | contribs) m (Changed protection level for "Terbinafine" ([Edit=Allow only autoconfirmed users] (expires 18:19, 21 January 2014 (UTC)) [Move=Allow only autoconfirmed users] (expires 18:19, 21 January 2014 (UTC)))) |
Gerald Chi- (talk | contribs) mNo edit summary |
||
| (16 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
| | |authorTag={{Ammu}} | ||
|genericName=Terbinafine | |||
|aOrAn=an | |||
|drugClass=[[antifungal]] | |||
|indicationType=treatment | |||
|indication=[[itching]], [[burning]], cracking and scaling in [[athlete's foot]] and [[jock itch]] | |||
|adverseReactions=[[diarrhea]], [[dyspepsia]], [[rash]], [[pruritis]], and [[urticaria]] | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |||
|fdaLIADAdult=* Cures most [[athlete's foot]] ([[tinea pedis]]) | |||
* Cures most jock itch (tinea cruris) and ringworm (tinea corporis) | |||
* Relieves itching, burning, cracking and scaling which accompany these conditions | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Terbinafine in adult patients. | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Terbinafine in adult patients. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Terbinafine in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Terbinafine in pediatric patients. | |||
|warnings=* For external use only | |||
* Do not use on nails or scalp in or near the mouth or the [[eyes]] for vaginal [[yeast]] infections | |||
* When using this product do not get into the eyes. If eye contact occurs, rinse thoroughly with water. | |||
* Stop use and ask a doctor if too much irritation occurs or gets worse. | |||
* Side effects occur. | |||
* Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away. | |||
|administration======Adults and Children 12 years and Older===== | |||
* Use the tip of the cap to break the seal and open the tube | |||
* wash the affected skin with soap and water and dry completely before applying for athlete's foot wear well-fitting, ventilated shoes. | |||
* Change shoes and socks at least once daily. | |||
:* between the toes only: apply twice a day (morning and night) for 1 week or as directed by a doctor. | |||
:* on the bottom or sides of the foot: apply twice a day (morning and night) for 2 weeks or as directed by a doctor. | |||
* For jock itch and ringworm: apply once a day (morning or night) for 1 week or as directed by a doctor. | |||
* Wash hands after each use | |||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| verifiedrevid = 457127366 | |||
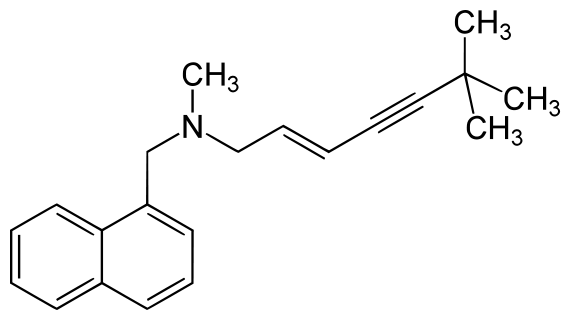
| IUPAC_name = [(2''E'')-6,6-dimethylhept-2-en-4-yn-1-yl](methyl)(naphthalen-1-ylmethyl)amine | |||
| image = Terbinafine.png | | image = Terbinafine.png | ||
<!--Clinical data--> | |||
| tradename = Lamisil | |||
| Drugs.com = {{drugs.com|monograph|terbinafine-hydrochloride}} | |||
| MedlinePlus = a699061 | |||
| pregnancy_category = B | |||
| legal_status = Low-strength preparations available without prescription | |||
| routes_of_administration = Oral, [[topical]] | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = Readily absorbed: 70–90% | |||
| protein_bound = >99% | |||
| metabolism = [[Hepatic]] | |||
| elimination_half-life = Highly variable | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 91161-71-6 | | CAS_number = 91161-71-6 | ||
| CAS_supplemental = 78628-80-5 | |||
| ATC_prefix = D01 | | ATC_prefix = D01 | ||
| ATC_suffix = AE15 | | ATC_suffix = AE15 | ||
| ATC_supplemental = {{ATC|D01|BA02}} | | ATC_supplemental = {{ATC|D01|BA02}} | ||
| PubChem = | | PubChem = 1549008 | ||
| DrugBank = | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| DrugBank = DB00857 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 1266005 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = G7RIW8S0XP | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D02375 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 822 | |||
| ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| ChEBI = 9448 | |||
<!--Chemical data--> | |||
| C=21 | H=25 | N=1 | | C=21 | H=25 | N=1 | ||
| molecular_weight = 291.43 g/mol | | molecular_weight = 291.43 g/mol | ||
| | | smiles = C(#C\C=C\CN(C)Cc2cccc1ccccc12)C(C)(C)C | ||
| | | InChI = 1/C21H25N/c1-21(2,3)15-8-5-9-16-22(4)17-19-13-10-12-18-11-6-7-14-20(18)19/h5-7,9-14H,16-17H2,1-4H3/b9-5+ | ||
| | | InChIKey = DOMXUEMWDBAQBQ-WEVVVXLNBZ | ||
| | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | | StdInChI = 1S/C21H25N/c1-21(2,3)15-8-5-9-16-22(4)17-19-13-10-12-18-11-6-7-14-20(18)19/h5-7,9-14H,16-17H2,1-4H3/b9-5+ | ||
| | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | | StdInChIKey = DOMXUEMWDBAQBQ-WEVVVXLNSA-N | ||
}} | }} | ||
{{ | |storage=* Store at controlled room temperature 20°-25°C (68°-77°F) | ||
|packLabel=[[File:Terbinafine01.jpg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
|alcohol=Alcohol-Terbinafine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
|brandNames=*ANTIFUNGAL ®<ref>{{Cite web | title =ANTIFUNGAL- terbinafine hydrochloride cream | url =http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=9daf3136-74b6-4410-8256-cbc9dd700f61 }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
}} | |||
[[Category:Antifungals]] | [[Category:Antifungals]] | ||
[[Category: | [[Category:Drugs]] | ||
Latest revision as of 11:04, 25 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Terbinafine (topical) is an antifungal that is FDA approved for the treatment of itching, burning, cracking and scaling in athlete's foot and jock itch. Common adverse reactions include diarrhea, dyspepsia, rash, pruritis, and urticaria.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Cures most athlete's foot (tinea pedis)
- Cures most jock itch (tinea cruris) and ringworm (tinea corporis)
- Relieves itching, burning, cracking and scaling which accompany these conditions
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Terbinafine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Terbinafine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Terbinafine (topical) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Terbinafine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Terbinafine in pediatric patients.
Contraindications
There is limited information regarding Terbinafine (topical) Contraindications in the drug label.
Warnings
- For external use only
- Do not use on nails or scalp in or near the mouth or the eyes for vaginal yeast infections
- When using this product do not get into the eyes. If eye contact occurs, rinse thoroughly with water.
- Stop use and ask a doctor if too much irritation occurs or gets worse.
- Side effects occur.
- Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Terbinafine (topical) Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Terbinafine (topical) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Terbinafine (topical) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Terbinafine (topical) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Terbinafine (topical) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Terbinafine (topical) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Terbinafine (topical) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Terbinafine (topical) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Terbinafine (topical) in geriatric settings.
Gender
There is no FDA guidance on the use of Terbinafine (topical) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Terbinafine (topical) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Terbinafine (topical) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Terbinafine (topical) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Terbinafine (topical) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Terbinafine (topical) in patients who are immunocompromised.
Administration and Monitoring
Administration
Adults and Children 12 years and Older
- Use the tip of the cap to break the seal and open the tube
- wash the affected skin with soap and water and dry completely before applying for athlete's foot wear well-fitting, ventilated shoes.
- Change shoes and socks at least once daily.
- between the toes only: apply twice a day (morning and night) for 1 week or as directed by a doctor.
- on the bottom or sides of the foot: apply twice a day (morning and night) for 2 weeks or as directed by a doctor.
- For jock itch and ringworm: apply once a day (morning or night) for 1 week or as directed by a doctor.
- Wash hands after each use
Monitoring
There is limited information regarding Terbinafine (topical) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Terbinafine (topical) and IV administrations.
Overdosage
There is limited information regarding Terbinafine (topical) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
Terbinafine (topical)
| |
| Systematic (IUPAC) name | |
| [(2E)-6,6-dimethylhept-2-en-4-yn-1-yl](methyl)(naphthalen-1-ylmethyl)amine | |
| Identifiers | |
| CAS number | 78628-80-5 |
| ATC code | D01 D01BA02 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 291.43 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | Readily absorbed: 70–90% |
| Protein binding | >99% |
| Metabolism | Hepatic |
| Half life | Highly variable |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
B |
| Legal status |
Low-strength preparations available without prescription |
| Routes | Oral, topical |
Mechanism of Action
There is limited information regarding Terbinafine (topical) Mechanism of Action in the drug label.
Structure
There is limited information regarding Terbinafine (topical) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Terbinafine (topical) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Terbinafine (topical) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Terbinafine (topical) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Terbinafine (topical) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Terbinafine (topical) How Supplied in the drug label.
Storage
- Store at controlled room temperature 20°-25°C (68°-77°F)
Images
Drug Images
{{#ask: Page Name::Terbinafine (topical) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Terbinafine (topical) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Terbinafine (topical) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Terbinafine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ANTIFUNGAL ®[1]
Look-Alike Drug Names
There is limited information regarding Terbinafine (topical) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.