Sandbox ap: Difference between revisions
Jump to navigation
Jump to search
(Created page with "==Management== {{familytree/start |summary=Acute Pancreatitis}} {{familytree | | | | | | | | | | | | | A01 |-|-|-|-|-|-|-|-|-|-|-|-|.| |A01='''Signs & symptoms''': severe abd...") |
(→Widget) |
||
| (44 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
== | ====Classification Gastritis== | ||
{| class="wikitable" | |||
!colspan="2" | Gastritis | |||
!Etiology | |||
!Gasstritis synonyms | |||
|- | |||
|colspan="2" | Non-atrophic | |||
| | |||
*Helicobacter pylori | |||
*Other factors | |||
| | |||
Superficial | |||
Diffuse antral gastritis (DAG) | |||
Chronic antral gastritis (CAG) | |||
Interstitial - follicular | |||
Hypersecretory | |||
Type B* | |||
|- | |||
|rowspan="4" |Atrophic | |||
|Autoimmune | |||
| | |||
*Autoimmunity | |||
| | |||
Type A* | |||
Diffuse corporal | |||
Pernicious anemia-associated | |||
|- | |||
|rowspan="3"|Multifocal atrophic | |||
|Helicobacter pylori | |||
|Type B*, type AB* | |||
|- | |||
|Dietary | |||
|Environmental | |||
|- | |||
|Environmental factors | |||
|Metaplastic | |||
|- | |||
|rowspan="7"| Special form | |||
|rowspan="4"| Chemical | |||
|Chemical irritation | |||
|Reactive | |||
|- | |||
| | |||
*Bile | |||
| | |||
*Reflux | |||
|- | |||
| | |||
*NSAIDs | |||
| | |||
*NSAID | |||
|- | |||
| | |||
*Other agents | |||
| | |||
*Type C* | |||
|- | |||
|Radiation | |||
|Radiation injury | |||
| | |||
|} | |||
==Risk assessment table== | |||
{ | {| | ||
! colspan="3" style="background:#4479BA; color: #FFFFFF;" align="center" + |Scoring criteria for risk assessment* | |||
|- | |||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Scoring system | |||
{{ | ! style="background:#4479BA; color: #FFFFFF;" align="center" + |Score | ||
! style="background:#4479BA; color: #FFFFFF;" align="center" + |Risk | |||
|- | |||
| rowspan="14" style="background:#DCDCDC;" align="center" + |'''IMPROVEDD Score'''<ref>{{cite journal|doi=10.1055/s-0037-160392910.1055/s-0037-1603929}}</ref> | |||
| style="background:#DCDCDC;" align="center" + | | |||
| style="background:#DCDCDC;" align="center" + |Predicted % VTE risk through 42 days | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |0 | |||
| style="background:#F5F5F5;" + |0.4% | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |1 | |||
| style="background:#F5F5F5;" + |0.6% | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |2 | |||
| style="background:#F5F5F5;" + |0.8% | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |3 | |||
| style="background:#F5F5F5;" + |1.2% | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |4 | |||
| style="background:#F5F5F5;" + |1.6% | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |5-10 | |||
| style="background:#F5F5F5;" + |2.2% | |||
|- | |||
| style="background:#DCDCDC;" align="center" + | | |||
| style="background:#DCDCDC;" align="center" + |Predicted % VTE risk through 77 days | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |0 | |||
| style="background:#F5F5F5;" + |0.5% | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |1 | |||
| style="background:#F5F5F5;" + |0.7% | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |2 | |||
| style="background:#F5F5F5;" + |1.0% | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |3 | |||
| style="background:#F5F5F5;" + |1.4% | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |4 | |||
| style="background:#F5F5F5;" + |1.9% | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |5-10 | |||
| style="background:#F5F5F5;" + |2.75 | |||
|- | |||
| rowspan="7" style="background:#DCDCDC;" align="center" + |'''IMPROVE score'''<ref name="pmid21436241">{{cite journal| author=Spyropoulos AC, Anderson FA, Fitzgerald G, Decousus H, Pini M, Chong BH et al.| title=Predictive and associative models to identify hospitalized medical patients at risk for VTE. | journal=Chest | year= 2011 | volume= 140 | issue= 3 | pages= 706-14 | pmid=21436241 | doi=10.1378/chest.10-1944 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=21436241 }} </ref> | |||
| style="background:#DCDCDC;" align="center" + | | |||
| style="background:#DCDCDC;" align="center" + |Predicted % VTE risk through 3 months | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |0 | |||
| style="background:#F5F5F5;" + |0.5% | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |1 | |||
| style="background:#F5F5F5;" + |1.0% | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |2 | |||
| style="background:#F5F5F5;" + |1.7% | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |3 | |||
| style="background:#F5F5F5;" + |3.1% | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |4 | |||
| style="background:#F5F5F5;" + |4% | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |5-8 | |||
| style="background:#F5F5F5;" + |11% | |||
|- | |||
| rowspan="2" style="background:#DCDCDC;" align="center" + | '''Padua Score'''<ref name="pmid20738765">{{cite journal| author=Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M et al.| title=A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. | journal=J Thromb Haemost | year= 2010 | volume= 8 | issue= 11 | pages= 2450-7 | pmid=20738765 | doi=10.1111/j.1538-7836.2010.04044.x | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=20738765 }} </ref> | |||
| style="background:#F5F5F5;" align="center" + |< 4 | |||
| style="background:#F5F5F5;" + |Low risk for VTE | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |≥ 4 | |||
| style="background:#F5F5F5;" + |High risk for VTE | |||
|- | |||
| rowspan="4" style="background:#DCDCDC;" align="center" + |'''Caprini score'''<ref name="pmid1754886">{{cite journal| author=Caprini JA, Arcelus JI, Hasty JH, Tamhane AC, Fabrega F| title=Clinical assessment of venous thromboembolic risk in surgical patients. | journal=Semin Thromb Hemost | year= 1991 | volume= 17 Suppl 3 | issue= | pages= 304-12 | pmid=1754886 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=1754886 }} </ref> | |||
| style="background:#F5F5F5;" align="center" + |0-1 | |||
| style="background:#F5F5F5;" + |Low risk of VTE | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |2 | |||
| style="background:#F5F5F5;" + |Moderate of VTE | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |3-4 | |||
| style="background:#F5F5F5;" + |High risk of VTE | |||
|- | |||
| style="background:#F5F5F5;" align="center" + |≥ 5 | |||
| style="background:#F5F5F5;" + |Highest risk for VTE | |||
|} | |||
==Images ILD== | |||
<gallery widths=200px> | |||
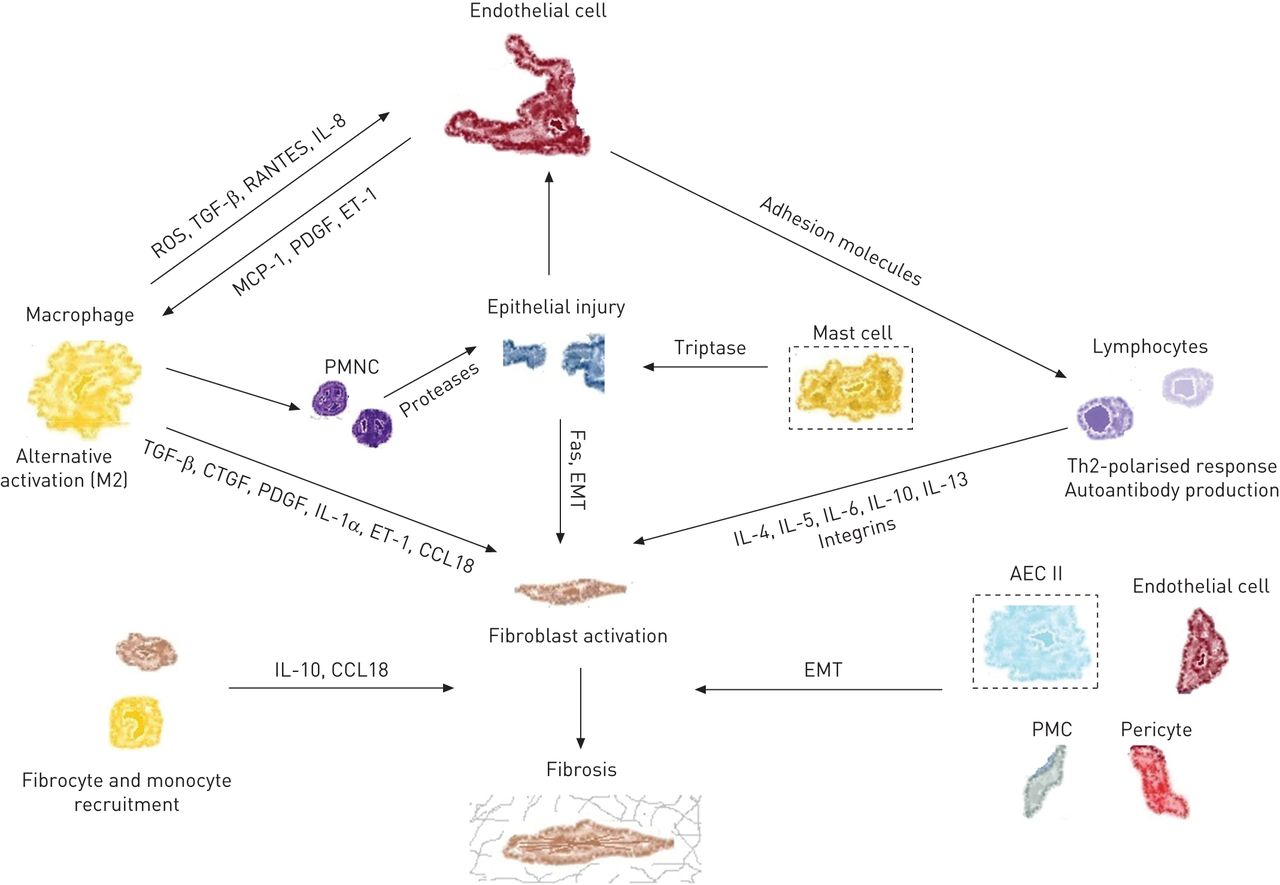
F2.large.jpg | Cellular Players and Molecules in IPF <br> [http://err.ersjournals.com/content/24/135/102.full<font size="-2">''Adapted from European Respiratory Review''</font>] | |||
</gallery> | |||
<gallery widths=200px> | |||
1-s2.0-S0272523112000044-gr6.jpg | Flow Chart for Lung Fibrosis Evaluation in ILD <br> [http://http://www.sciencedirect.com/science/article/pii/S0272523112000044/ <font size="-2">''Adapted from Clinics in Chest Medicine''</font>] | |||
</gallery> | |||
==Widget== | |||
<div class="nomobile"> | |||
<div style="position:right; width:50%; float:right; background-color:#d0d0d0; border-radius: 10px;"><span style="position:right; float:right; width: 100%;"><center>'''Tweets by NEJM!'''<hr>{{#Widget:NEJM}}</center> | |||
</span></div> | |||
</div> | |||
<br style="clear:right" /> | |||
==References== | |||
Latest revision as of 16:03, 16 May 2018
==Classification Gastritis
| Gastritis | Etiology | Gasstritis synonyms | |
|---|---|---|---|
| Non-atrophic |
|
Superficial Diffuse antral gastritis (DAG) Chronic antral gastritis (CAG) Interstitial - follicular Hypersecretory Type B* | |
| Atrophic | Autoimmune |
|
Type A* Diffuse corporal Pernicious anemia-associated |
| Multifocal atrophic | Helicobacter pylori | Type B*, type AB* | |
| Dietary | Environmental | ||
| Environmental factors | Metaplastic | ||
| Special form | Chemical | Chemical irritation | Reactive |
|
| ||
|
| ||
|
| ||
| Radiation | Radiation injury | ||
Risk assessment table
| Scoring criteria for risk assessment* | ||
|---|---|---|
| Scoring system | Score | Risk |
| IMPROVEDD Score[1] | Predicted % VTE risk through 42 days | |
| 0 | 0.4% | |
| 1 | 0.6% | |
| 2 | 0.8% | |
| 3 | 1.2% | |
| 4 | 1.6% | |
| 5-10 | 2.2% | |
| Predicted % VTE risk through 77 days | ||
| 0 | 0.5% | |
| 1 | 0.7% | |
| 2 | 1.0% | |
| 3 | 1.4% | |
| 4 | 1.9% | |
| 5-10 | 2.75 | |
| IMPROVE score[2] | Predicted % VTE risk through 3 months | |
| 0 | 0.5% | |
| 1 | 1.0% | |
| 2 | 1.7% | |
| 3 | 3.1% | |
| 4 | 4% | |
| 5-8 | 11% | |
| Padua Score[3] | < 4 | Low risk for VTE |
| ≥ 4 | High risk for VTE | |
| Caprini score[4] | 0-1 | Low risk of VTE |
| 2 | Moderate of VTE | |
| 3-4 | High risk of VTE | |
| ≥ 5 | Highest risk for VTE | |
Images ILD
-
Cellular Players and Molecules in IPF
Adapted from European Respiratory Review
-
Flow Chart for Lung Fibrosis Evaluation in ILD
Adapted from Clinics in Chest Medicine
Widget
Tweets by NEJM
References
- ↑ . doi:10.1055/s-0037-160392910.1055/s-0037-1603929. Missing or empty
|title=(help) - ↑ Spyropoulos AC, Anderson FA, Fitzgerald G, Decousus H, Pini M, Chong BH; et al. (2011). "Predictive and associative models to identify hospitalized medical patients at risk for VTE". Chest. 140 (3): 706–14. doi:10.1378/chest.10-1944. PMID 21436241.
- ↑ Barbar S, Noventa F, Rossetto V, Ferrari A, Brandolin B, Perlati M; et al. (2010). "A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score". J Thromb Haemost. 8 (11): 2450–7. doi:10.1111/j.1538-7836.2010.04044.x. PMID 20738765.
- ↑ Caprini JA, Arcelus JI, Hasty JH, Tamhane AC, Fabrega F (1991). "Clinical assessment of venous thromboembolic risk in surgical patients". Semin Thromb Hemost. 17 Suppl 3: 304–12. PMID 1754886.