Medrogestone: Difference between revisions
No edit summary |
m (Protected "Medrogestone": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (One intermediate revision by one other user not shown) | |||
| Line 45: | Line 45: | ||
==Overview== | ==Overview== | ||
'''Medrogestone''' ([[International Nonproprietary Name|INN]]; trade names '''Colpro(ne)''' by [[Wyeth]] and '''Prothil''' by [[Solvay (company)|Solvay]]) is a [[progestin]], a synthetic [[pharmaceutical drug|drug]] with similar effects as [[progesterone]], | '''Medrogestone''' ([[International Nonproprietary Name|INN]]; trade names '''Colpro(ne)''' by [[Wyeth]] and '''Prothil''' by [[Solvay (company)|Solvay]]) is a [[progestin]], a synthetic [[pharmaceutical drug|drug]] with similar effects as [[progesterone]], a hormone involved in the [[menstrual cycle]] and [[pregnancy]]. As of 2010, it is no longer available in Germany or Austria. | ||
==Indications== | ==Indications== | ||
In the past, medrogestone was used in the treatment of [[endometrial cancer]] and in some regimens for [[breast cancer]], and, in men, for [[benign prostatic hyperplasia]]. It still finds use in the treatment of [[amenorrhea]] and as the progestin component in certain forms of [[Hormone replacement therapy (menopause)|menopausal hormone replacement therapy]]. | In the past, medrogestone was used in the treatment of [[endometrial cancer]] and in some regimens for [[breast cancer]], and, in men, for [[benign prostatic hyperplasia]]. It still finds use in the treatment of [[amenorrhea]] and as the progestin component in certain forms of [[Hormone replacement therapy (menopause)|menopausal hormone replacement therapy]]. | ||
==Contraindications== | ==Contraindications== | ||
[[Intrahepatic cholestasis of pregnancy]] (acute or in history), [[vaginal bleeding]] of unknown origin, and severe diseases of the liver such as tumours are absolute contraindications for medrogestone. Relative contraindications include a history of [[jaundice]] or [[itch]]ing in pregnancy or [[gestational pemphigoid]]. | [[Intrahepatic cholestasis of pregnancy]] (acute or in history), [[vaginal bleeding]] of unknown origin, and severe diseases of the liver such as tumours are absolute contraindications for medrogestone. Relative contraindications include a history of [[jaundice]] or [[itch]]ing in pregnancy or [[gestational pemphigoid]]. | ||
===Pregnancy and lactation=== | ===Pregnancy and lactation=== | ||
Medrogestone is contraindicated during pregnancy because progestogens are associated with risks for the foetus in animals and humans. | Medrogestone is contraindicated during pregnancy because progestogens are associated with risks for the foetus in animals and humans. Studies in pregnant rabbits have shown [[skeletal]] deformations under 3 mg medrogestone per kilogram body weight but not under 1 mg/kg. Typical therapeutic doses are between 0.1 and 0.25 mg/kg. | ||
It is not known whether medrogestone passes into [[breast milk]], but it is to be expected given its [[lipophilicity]] and studies with chemically related progestins. | It is not known whether medrogestone passes into [[breast milk]], but it is to be expected given its [[lipophilicity]] and studies with chemically related progestins. | ||
Latest revision as of 16:39, 20 August 2015
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 100% |
| Metabolism | Hydroxylation and glucuronidation |
| Elimination half-life | 4 to 5 hours |
| Excretion | Renal and fecal, as metabolites |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C23H32O2 |
| Molar mass | 340.5 g/mol |
| 3D model (JSmol) | |
| |
|
WikiDoc Resources for Medrogestone |
|
Articles |
|---|
|
Most recent articles on Medrogestone Most cited articles on Medrogestone |
|
Media |
|
Powerpoint slides on Medrogestone |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Medrogestone at Clinical Trials.gov Clinical Trials on Medrogestone at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Medrogestone
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Medrogestone Discussion groups on Medrogestone Patient Handouts on Medrogestone Directions to Hospitals Treating Medrogestone Risk calculators and risk factors for Medrogestone
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Medrogestone |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Medrogestone (INN; trade names Colpro(ne) by Wyeth and Prothil by Solvay) is a progestin, a synthetic drug with similar effects as progesterone, a hormone involved in the menstrual cycle and pregnancy. As of 2010, it is no longer available in Germany or Austria.
Indications
In the past, medrogestone was used in the treatment of endometrial cancer and in some regimens for breast cancer, and, in men, for benign prostatic hyperplasia. It still finds use in the treatment of amenorrhea and as the progestin component in certain forms of menopausal hormone replacement therapy.
Contraindications
Intrahepatic cholestasis of pregnancy (acute or in history), vaginal bleeding of unknown origin, and severe diseases of the liver such as tumours are absolute contraindications for medrogestone. Relative contraindications include a history of jaundice or itching in pregnancy or gestational pemphigoid.
Pregnancy and lactation
Medrogestone is contraindicated during pregnancy because progestogens are associated with risks for the foetus in animals and humans. Studies in pregnant rabbits have shown skeletal deformations under 3 mg medrogestone per kilogram body weight but not under 1 mg/kg. Typical therapeutic doses are between 0.1 and 0.25 mg/kg.
It is not known whether medrogestone passes into breast milk, but it is to be expected given its lipophilicity and studies with chemically related progestins.
Adverse effects
Medrogestone seldom produces adverse effects, all of which are typical of progestogens. They include lack of appetite, nausea, headache, dizziness, and depression.
Overdose
The acute toxicity of the drug is low. Overdose causes only harmless side-effects such as nausea and vaginal bleeding. The Template:LD50 has been found to range between 500 mg/kg in dogs and over 3000 mg/kg in rats. Chronic toxicity has been examined in animals, but nothing but the typical adverse effects of progestogens, and reduction of prostatic weight in Rhesus Monkeys, have been found. Accidental intake of the drug, including in children, is not dangerous.
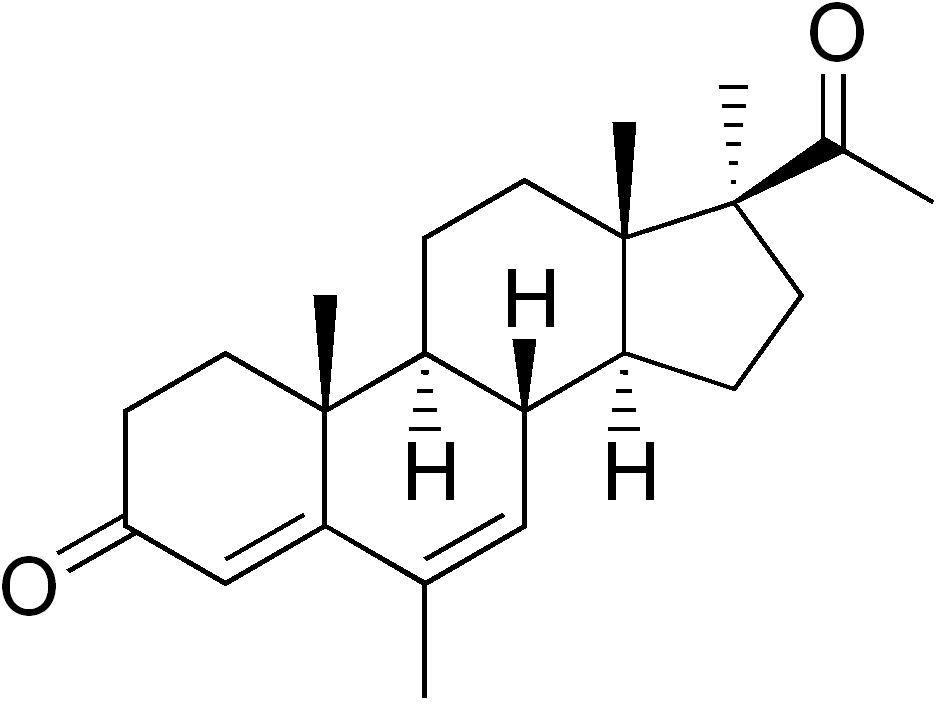
Chemical properties
Medrogestone is a steroid. More specifically, it is a derivative of pregna-4,6-diene structurally related to the progestin chlormadinone acetate and the antiandrogen cyproterone acetate. As is frequently found in other synthetic steroid hormones, medrogestone possesses a lipophilic group at position 6. However in contrast to chlormadinone acetate and cyproterone acetate or to fluocinolone that contain a chlorine or fluorine respectively at position 6, medrogestone contains a methyl substituent at this position. The methyl in position 17 is unusual for a steroid, as many such drugs carry an oxygen atom in that position.
Pharmacology
Pharmacokinetics
The drug is absorbed quickly and completely from the gut and reaches peak plasma concentrations after about one to four hours. Unlike many other steroids it binds neither to transcortin (corticosteroid-binding globulin, CBG, which binds progesterone nor to sex hormone-binding globulin (SHBG), but to albumin.Medrogestone itself cannot be excreted. The substance is hydroxylised and glucuronidised in the liver, and the resulting metabolites are eliminated via urine and faeces.
Pharmacodynamics
The profile of medrogestone is similar to the natural hormone progesterone. It has pronounced progestogenic effects and opposes the proliferative effects of estrogen in the utereus, but lacks anabolic, androgenic, estrogenic and corticoid activity. In extremely high doses it is an androgen antagonist (in 2500-fold therapeutic doses) as well as an antigonadotropin.
Interactions
Enzyme inducers such as barbiturates, phenylbutazone, phenytoin, ampicillin or tetracyclines are expected to reduce plasma concentrations of medrogestone, but no systematic research has been done.