|
|
| (16 intermediate revisions by 5 users not shown) |
| Line 1: |
Line 1: |
| {| class="infobox" style="margin: 0 0 3em 3em; border: 1px solid #696969; float: right; width: 250px;" cellpadding="0" cellspacing="0"; | | {{Drugbox |
| ! style="padding: 0 5px; font-size: 100%; background:#F8F8FF;" align=center | [[Metoprolol|{{fontcolor|#2B3B44|Metoprolol}}]]
| | | verifiedrevid = 459444186 |
| |-
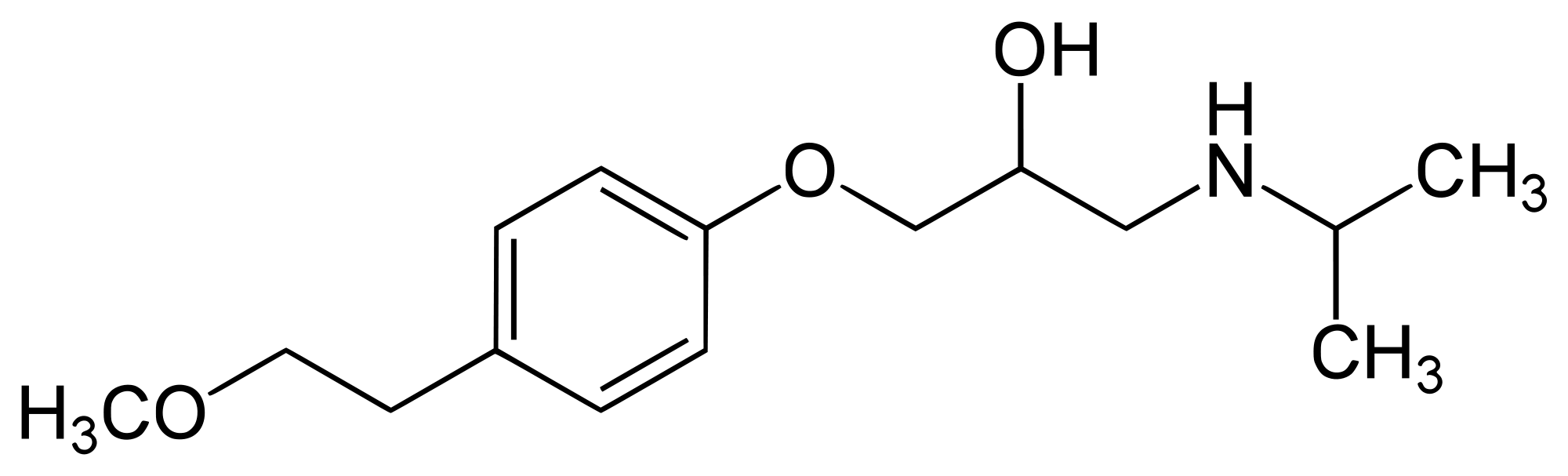
| | | IUPAC_name = (''RS'')-1-(Isopropylamino)-3-[4-(2-methoxyethyl)phenoxy]propan-2-ol |
| ! style="padding: 0 5px; font-size: 85%; background:#F5F5F5" align=left | [[Lopressor|{{fontcolor|#2B3B44|Lopressor (metoprolol tartrate) tablet}}]] | | | image = 2000px-Metoprolol structure.svg.png |
| |- | | | drug_name = Metoprolol |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor indications and usage|Indications and Usage]]
| | |
| |- | | <!--Clinical data--> |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor dosage and administration|Dosage and Administration]]
| | | tradename = Lopressor, Toprol-xl |
| |-
| | | Drugs.com = {{drugs.com|monograph|metoprolol-succinate}} |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor dosage forms and strengths|Dosage Forms and Strengths]] | | | MedlinePlus = a682864 |
| |- | | | licence_US = Metoprolol |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor contraindications|Contraindications]]
| | | pregnancy_AU = C |
| |-
| | | pregnancy_US = C |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor warnings and precautions|Warnings and Precautions]]
| | | legal_status = Rx-only |
| |-
| | | routes_of_administration = Oral, [[Intravenous|IV]] |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor adverse reactions|Adverse Reactions]]
| | |
| |- | | <!--Pharmacokinetic data--> |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor drug interactions|Drug Interactions]]
| | | bioavailability = 12% |
| |-
| | | metabolism = [[Liver|Hepatic]] via [[CYP2D6]], [[CYP3A4]] |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor use in specific populations|Use in Specific Populations]] | | | elimination_half-life = 3-7 hours |
| |- | | | excretion = [[Kidney|Renal]] |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor overdosage|Overdosage]]
| | |
| |- | | <!--Identifiers--> |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor description|Description]]
| | | CAS_number_Ref = {{cascite|correct|??}} |
| |- | | | CAS_number = 51384-51-1 |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor clinical pharmacology|Clinical Pharmacology]]
| | | ATC_prefix = C07 |
| |- | | | ATC_suffix = AB02 |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor nonclinical toxicology|Nonclinical Toxicology]]
| | | PubChem = 4171 |
| |- | | | IUPHAR_ligand = 553 |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor clinical studies|Clinical Studies]]
| | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} |
| |- | | | DrugBank = DB00264 |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor how supplied storage and handling|How Supplied/Storage and Handling]]
| | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
| |- | | | ChemSpiderID = 4027 |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor patient counseling information|Patient Counseling Information]]
| | | UNII_Ref = {{fdacite|correct|FDA}} |
| |- | | | UNII = GEB06NHM23 |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Lopressor labels and packages|Labels and Packages]]
| | | KEGG_Ref = {{keggcite|correct|kegg}} |
| |- | | | KEGG = D02358 |
| ! style="padding: 0 5px; font-size: 85%; background:#F5F5F5" align=left | [[Toprol XL|{{fontcolor|#2B3B44|Toprol XL (metoprolol succinate) tablet, extended release}}]]
| | | ChEBI_Ref = {{ebicite|correct|EBI}} |
| |- | | | ChEBI = 6904 |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL indications and usage|Indications and Usage]]
| | | ChEMBL_Ref = {{ebicite|correct|EBI}} |
| |-
| | | ChEMBL = 13 |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL dosage and administration|Dosage and Administration]] | | |
| |-
| | <!--Chemical data--> |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL dosage forms and strengths|Dosage Forms and Strengths]]
| | | C=15 | H=25 | N=1 | O=3 |
| |- | | | molecular_weight = 267.364 [[gram|g]]/[[Mole (unit)|mol]] |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL contraindications|Contraindications]]
| | | smiles = O(c1ccc(cc1)CCOC)CC(O)CNC(C)C |
| |- | | | InChI = 1/C15H25NO3/c1-12(2)16-10-14(17)11-19-15-6-4-13(5-7-15)8-9-18-3/h4-7,12,14,16-17H,8-11H2,1-3H3 |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL warnings and precautions|Warnings and Precautions]]
| | | InChIKey = IUBSYMUCCVWXPE-UHFFFAOYAN |
| |-
| | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL adverse reactions|Adverse Reactions]]
| | | StdInChI = 1S/C15H25NO3/c1-12(2)16-10-14(17)11-19-15-6-4-13(5-7-15)8-9-18-3/h4-7,12,14,16-17H,8-11H2,1-3H3 |
| |- | | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL drug interactions|Drug Interactions]]
| | | StdInChIKey = IUBSYMUCCVWXPE-UHFFFAOYSA-N |
| |- | | | melting_point = 120 |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL use in specific populations|Use in Specific Populations]]
| | }} |
| |-
| |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL overdosage|Overdosage]]
| |
| |-
| |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL description|Description]]
| |
| |-
| |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL clinical pharmacology|Clinical Pharmacology]]
| |
| |-
| |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL nonclinical toxicology|Nonclinical Toxicology]]
| |
| |-
| |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL clinical studies|Clinical Studies]]
| |
| |-
| |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL how supplied storage and handling|How Supplied/Storage and Handling]]
| |
| |-
| |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL patient counseling information|Patient Counseling Information]]
| |
| |- | |
| ! style="font-size: 80%; padding: 0 5px; background: #DCDCDC" align=left | [[Toprol XL labels and packages|Labels and Packages]]
| |
| |- | |
| ! style="padding: 0 5px; font-size: 85%; background:#F5F5F5" align=left | [[Metoprolol (patient information)|{{fontcolor|#2B3B44|Patient Information}}]]
| |
| |- | |
| ! style="padding: 0 5px; font-size: 85%; background:#F5F5F5" align=left | [http://clinicaltrials.gov/search/open/condition={{urlencode:{{#if:{{{1|}}}|{{{1}}}|{{PAGENAME}}}}}} {{fontcolor|#2B3B44|Clinical Trials}}]
| |
| |- | |
| |} | |