Acetylcysteine (injection): Difference between revisions
m (Protected "Acetylcysteine": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
Gerald Chi- (talk | contribs) mNo edit summary |
||
| (51 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
| | |authorTag={{DB}} | ||
| | |genericName=Acetylcysteine | ||
| | |aOrAn=an | ||
| | |drugClass=antioxidant, respiratory system agent | ||
| | |indicationType=treatment | ||
| | |indication=acetaminophen overdose, adjuvant therapy for patients with abnormal, viscid, or inspissated mucous secretions in such conditions as [[chronic bronchopulmonary disease]] ([[chronic emphysema]], [[emphysema]] with [[bronchitis]], [[chronic asthmatic bronchitis]], [[tuberculosis]], [[bronchiectasis]] and [[primary amyloidosis]] of the lung), [[acute bronchopulmonary disease]] ([[pneumonia]], [[bronchitis]], [[tracheobronchitis]]), pulmonary complications of [[cystic fibrosis]], [[tracheostomy]] care, pulmonary complications associated with surgery, use during anesthesia, post-traumatic chest conditions, [[atelectasis]] due to mucous obstruction, diagnostic bronchial studies ([[bronchograms]], [[bronchospirometry]], and bronchial wedge catheterization) | ||
| | |adverseReactions=[[rash]], [[urticaria]]/[[facial flushing]] and [[pruritus]], [[diarrhea]], [[nausea]], [[vomiting]] | ||
| | |blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | ||
| | |blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | ||
| | |||
* Content | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
| | |fdaLIADAdult======Acetaminophen overdose===== | ||
*Acetylcysteine (injection) is an antidote for acetaminophen overdose indicated to prevent or lessen hepatic injury after ingestion of a potentially hepatotoxic quantity of acetaminophen. Overdose incidences are divided into two types; Acute Ingestion or Repeated Supratherapeutic Ingestion (RSI). | |||
*On admission for suspected acetaminophen overdose, a serum blood sample should be drawn at least 4 hours after ingestion to determine the acetaminophen level and will serve as a basis for determining the need for treatment with acetylcysteine. If the patient presents after 4 hours post-ingestion, the serum acetaminophen sample should be determined immediately. | |||
{{ | *Acetylcysteine Injection should be administered within 8 hours from acetaminophen ingestion for maximal protection against hepatic injury for patients whose serum acetaminophen levels fall above the "possible" toxicity line on the Rumack-Matthew nomogram (line connecting 150 mcg/mL at 4 hours with 37.5 mcg/mL at 12 hours); [see Acetaminophen Assays – Interpretation and Methodology]. If the time of ingestion is unknown, or the serum acetaminophen level is not available, cannot be interpreted, or is not available within the 8 hour time interval from acetaminophen ingestion, Acetylcysteine Injection should be administered immediately if 24 hours or less have elapsed from the reported time of ingestion of an overdose of acetaminophen, regardless of the quantity reported to have been ingested. | ||
{{ | *The [[aspartate aminotransferase]] (AST, SGOT), [[alanine aminotranferase]] (ALT, SGPT), [[bilirubin]], [[prothrombin time]], [[creatinine]], blood urea nitrogen (BUN), blood glucose, and electrolytes also should be determined in order to monitor hepatic and renal function and electrolyte and fluid balance. | ||
*NOTE: The critical ingestion-treatment interval for maximal protection against severe hepatic injury is between 0 to 8 hours. Efficacy diminishes progressively after 8 hours and treatment initiation between 15 and 24 hours post-ingestion of acetaminophen yields limited efficacy. However, it does not appear to worsen the condition of patients and it should not be withheld, since the reported time of ingestion may not be correct. | |||
'''Acetaminophen Assays Interpretation and Methodology – Acute Ingestion''' | |||
*The acute ingestion of acetaminophen in quantities of 150 mg/kg or greater may result in hepatic toxicity. However, the reported history of the quantity of a drug ingested as an overdose is often inaccurate and is not a reliable guide to therapy of the overdose. Therefore, plasma or serum acetaminophen concentrations, determined as early as possible, but no sooner than four hours following an acute overdose, are essential in assessing the potential risk of [[hepatotoxicity]]. If an assay for acetaminophen cannot be obtained, it is necessary to assume that the overdose is potentially toxic. | |||
'''Interpretation of Acetaminophen Assays''' | |||
*When results of the plasma acetaminophen assay are available, refer to the nomogram in Figure 1 to determine if plasma concentration is in the potentially toxic range. Values above the line connecting 200 mcg/mL at 4 hours with 50 mcg/mL at 12 hours (probable line) are associated with a probability of hepatic toxicity if an antidote is not administered. | |||
*If the predetoxification plasma level is above the line connecting 150 mcg/mL at 4 hours with 37.5 mcg/mL at 12 hours (possible line), continue with maintenance doses of acetylcysteine. It is better to err on the safe side and thus this line, defining possible toxicity, is plotted 25% below the line defining probable toxicity. | |||
*If the predetoxification plasma level is below the line connecting 150 mcg/mL at 4 hours with 37.5 mcg/mL at 12 hours (possible line), there is minimal risk of hepatic toxicity, and Acetylcysteine treatment may be discontinued. | |||
*Estimating Potential for [[Hepatotoxicity]]: The following depiction of the Rumack- Matthew nomogram has been developed to estimate the probability that plasma levels in relation to intervals post-ingestion will result in hepatotoxicity. | |||
*The Rumack-Matthew nomogram may underestimate the risk for hepatotoxicity in some patients with risk factors such as chronic alcoholism, malnutrition, or CYP2E1 enzyme inducing drugs (e.g., isoniazid). | |||
'''Figure 1. Rumack-Matthew Nomogram:''' | |||
[[File:Acetylcysteine fig 1.jpg|600px|thumbnail|left]] | |||
{{clear}} | |||
*Figure 1. Michael J Hodgman, Alexander R Garrard, A Review of Acetaminophen Poisoning. Crit Care Clin. 28 (2012) 499-516. Stephen J. Wolf, Kennon Heard, et.al, Clinical Policy: Critical Issues in the Management of Patients Presenting to the Emergency Department with Acetaminophen Overdose. Ann Emerg Med. 2007:50:292-313. | |||
'''Acetaminophen Assays Interpretation and Methodology – Repeated Supratherapeutic Ingestion''' | |||
*Repeated Supratherapeutic Ingestion (RSI) is defined as ingestion of acetaminophen at doses higher than those recommended for extended periods of time. The nomogram does not apply to patients with RSI. Treatment is based on the acetaminophen and elevated AST/ALT levels indicative of potential toxicity due to acetaminophen. For specific treatment information regarding the clinical management of repeated supratherapeutic acetaminophen overdose, please contact your regional poison center at 1-800-222-1222, or alternatively, a special health professional assistance line for acetaminophen overdose at 1-800-525-6115. | |||
'''Figure 2. Acetylcysteine Injection Treatment Flow Chart''' | |||
[[File:Acetylcysteine fig 2.jpg|600px|thumbnail|left]] | |||
{{clear}} | |||
*1Acetaminophen levels drawn less than 4 hours post-ingestion may be misleading. | |||
*2With an extended-release preparation, an acetaminophen level drawn less than 8 hours post-ingestion may be misleading. Draw a second level at 4 to 6 hours after the initial level. If either falls above the toxicity line, acetylcysteine treatment should be initiated. | |||
*3Acetylcysteine may be withheld until acetaminophen assay results are available as long as initiation of treatment is not delayed beyond 8 hours post-ingestion. If more than 8 hours post-ingestion, start acetylcysteine treatment immediately. | |||
'''Dosing Information''' | |||
*The total dose of Acetylcysteine Injection is 300 mg/kg given as 3 separate doses and administered over a total of 21 hours. Please refer to the guidelines below for dose preparation based upon patient weight. The total volume administered should be adjusted for patients less than 40 kg and for those requiring fluid restriction (see Tables 1 and 2). | |||
'''Administration Instructions (Three-Bag Method: Loading, Second and Third Dose)''' | |||
*Dosing for Patients who weigh 5 kg to 20 kg (Table 1): | |||
*Loading Dose: 150 mg/kg diluted in 3 mL/kg of diluent* administered over 1 hr | |||
*Second Dose: 50 mg/kg diluted in 7 mL/kg of diluent* administered over 4 hrs | |||
*Third Dose: 100 mg/kg diluted in 14 mL/kg of diluent* administered over 16 hrs | |||
[[File:Acetylcysteine t 1.png|600px|thumbnail|left]] | |||
{{clear}} | |||
*See also Section 2.2 Volume Adjustment: Patients less than 40 kg and Requiring Fluid Restriction | |||
*Dosing for patients who weigh 21 kg to 40 kg (Table 2): | |||
*Loading Dose: 150 mg/kg diluted in 100 mL of diluent* administered over 1 hr | |||
*Second Dose: 50 mg/kg diluted in 250 mL of diluent* administered over 4 hrs | |||
*Third Dose: : 100 mg/kg diluted in 500 mL of diluent* administered over 16 hrs | |||
[[File:Acetylcysteine t 2.png|600px|thumbnail|left]] | |||
{{clear}} | |||
*See also Section 2.2 Volume Adjustment: Patients less than 40 kg and Requiring Fluid Restriction. | |||
*Dosing for patients who weigh 41 kg to 100 kg (Table 3): | |||
*Loading Dose: 150 mg/kg diluted in 200 mL of diluent* administered over 1 hr | |||
*Second Dose: 50 mg/kg diluted in 500 mL of diluent* administered over 4 hrs | |||
*Third Dose: 100 mg/kg diluted in 1,000 mL of diluent administered over 16 hrs | |||
[[File:Acetylcysteine t 3.png|600px|thumbnail|left]] | |||
{{clear}} | |||
'''Patients Weighing More Than 100 kg''' | |||
*No specific studies have been conducted to evaluate the use of or necessity of dosing adjustments in patients weighing over 100 kg. Limited information is available regarding the dosing requirements of patients that weigh more than 100 kg. The dose of Acetylcysteine Injection recommended in these patients should be a loading dose of 15,000 mg infused over a period of one hour followed by a first maintenance dose of 5,000 mg over 4 hours and a second maintenance dose of 10,000 mg over 16 hours (Table 3). | |||
'''Continued Therapy beyond 21 Hours''' | |||
*While there is no clinical trial data to support infusions beyond 21 hours there is literature that supports continued infusion of acetylcysteine in some rare instances. In cases of suspected massive overdose, or with concomitant ingestion of other substances, or in patients with preexisting liver disease, the absorption and/or the half-life of acetaminophen may be prolonged, in such cases consideration should be given to the need for continued infusion of N-acetylcysteine beyond 21 hours. Acetaminophen levels and ALT/AST & INR should be checked before the end of the 21-hour infusion. If acetaminophen levels are still detectable, or in cases in which the ALT/AST are still increasing or the INR remains elevated, the infusion should be continued, and the treating physician should contact a US regional poison center at 1-800-222-1222, or alternatively, a “special health professional assistance line for acetaminophen overdose” at 1-800-525-6115 for assistance with dosing recommendations. | |||
'''Volume Adjustment: Patients less than 40 kg and Requiring Fluid Restriction''' | |||
*The total volume administered should be adjusted for patients less than 40 kg and for those requiring fluid restriction. To avoid fluid overload, the volume of diluent should be reduced as clinically needed. If the volume of the infusion is not adjusted, fluid overload can occur, potentially resulting in hyponatremia, seizure and death. | |||
*As Acetylcysteine Injection is hyperosmolar (2600 mOsmol/L), caution is advised when the diluent volume is decreased as the hyperosmolarity of the solution is increased. See Table 4 below for examples. | |||
[[File:Acetylcysteine t 4.png|600px|thumbnail|left]] | |||
{{clear}} | |||
*Single dose vial, preservative-free, discard unused portion. If vial was previously opened, do not use for intravenous administration. | |||
*Stability studies indicate that the diluted solution is stable for 24 hours at controlled room temperature. | |||
*Note: The color of Acetylcysteine Injection may turn from essentially colorless to a slight pink or purple once the stopper is punctured. The color change does not affect the quality of the product. | |||
'''Renal Impairment''' | |||
*No data are available to determine if a dose adjustment in patients with moderate or severe renal impairment is required. | |||
'''Hepatic Impairment''' | |||
*Although there was a threefold increase in acetylcysteine plasma concentrations in patients with [[hepatic cirrhosis]], no data are available to determine if a dose adjustment in these patients is required. The published medical literature does not indicate that the dose of acetylcysteine in patients with hepatic impairment should be reduced. | |||
'''DOSAGE FORMS & STRENGTHS''' | |||
*Each single dose vial contains 6g/30mL (200 mg/mL) of Acetylcysteine. Acetylcysteine Injection is sterile and can be used for intravenous administration. | |||
=====Administration of anesthesia for procedure===== | |||
* Dosing Information | |||
:*Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily | |||
:*Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette | |||
:*Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours | |||
:*Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter | |||
:*Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter | |||
=====Atelectasis, Due to mucous obstruction===== | |||
* Dosing Information | |||
:*Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily | |||
:*Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette | |||
:*Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours | |||
:*Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter | |||
:*Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter | |||
=====Bronchopulmonary disease, acute===== | |||
* Dosing Information | |||
:*Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily | |||
:*Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette | |||
:*Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours | |||
:*Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter | |||
:*Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter | |||
====Complication of surgical procedure - Respiratory complication==== | |||
* Dosing Information | |||
:*Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily | |||
:*Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette | |||
:*Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours | |||
:*Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter | |||
:*Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter | |||
====Cystic fibrosis, Pulmonary complications; Adjunct==== | |||
* Dosing Information | |||
:*Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily | |||
:*Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette | |||
:*Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours | |||
:*Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter | |||
:*Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter | |||
====Diagnostic procedure on lower respiratory tract==== | |||
* Dosing Information | |||
:*Diagnostic bronchial studies: 1 to 2 mL of the 20% solution or 2 to 4 mL of the 10% solution 2 or 3 times prior to the procedure by nebulization or by instillation intratracheally | |||
====Disease of respiratory system, chronic==== | |||
* Dosing Information | |||
:*Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily | |||
:*Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette | |||
:*Disease of respiratory system, chronic: direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours | |||
:*Disease of respiratory system, chronic: percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter | |||
:*Disease of respiratory system, chronic: intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter | |||
====Tracheostomy care==== | |||
* Dosing Information | |||
:*Nebulization into tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily | |||
:*Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours | |||
:*Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter | |||
:*Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter | |||
|offLabelAdultGuideSupport=* There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | |||
|offLabelAdultNoGuideSupport=* There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | |||
|fdaLIADPed======Acetaminophen overdose===== | |||
* Dosing Information | |||
:*Oral: initial, 140 mg/kg ORALLY, then 70 mg/kg every 4 hours for 17 doses starting 4 hours after loading dose | |||
:*IV: body weight 5 to 20 kg: loading dose, 150 mg/kg in 3 mL/kg of compatible solution (D5W, 0.45%NaCl, or sterile water for injection) IV over 60 minutes, then 50 mg/kg in 7 mL/kg of compatible solution IV over 4 hours, followed by 100 mg/kg in 14 mL/kg of compatible solution IV over 16 hours; may continue beyond 21 hours if clinically indicated; adjust the total volume administered for patients requiring fluid restriction as clinically necessary | |||
:*IV: body weight 21 kg to 40 kg: loading dose, 150 mg/kg in 100 mL of compatible solution (D5W, 0.45%NaCl, or sterile water for injection) IV over 60 minutes, then 50 mg/kg in 250 mL of compatible solution IV over 4 hours, followed by 100 mg/kg in 500 mL of compatible solution IV over 16 hours; may continue beyond 21 hours if clinically indicated; adjust the total volume administered for patients less than 40 kg or requiring fluid restriction as clinically necessary | |||
:*IV: body weight 41 to 100 kg, loading dose, 150 mg/kg in 200 mL of compatible solution (D5W, 0.45%NaCl, or sterile water for injection) IV over 60 minutes, then 50 mg/kg in 500 mL of compatible solution IV over 4 hours, followed by 100 mg/kg in 1000 mL of solution IV over 16 hours; may continue beyond 21 hours if clinically indicated; adjust the total volume administered for patients requiring fluid restriction as clinically necessary [3] | |||
:*IV: body weight over 100 kg, loading dose, 15,000 mg in 200 mL of compatible solution (D5W, 0.45%NaCl, or sterile water for injection) IV over 60 minutes, then 5000 mg in 500 mL of compatible solution IV over 4 hours, followed by 10,000 mg in 1000 mL of solution IV over 16 hours; may continue beyond 21 hours if clinically indicated; adjust the total volume administered for patients requiring fluid restriction as clinically necessary | |||
=====Administration of anesthesia for procedure===== | |||
* Dosing Information | |||
:*Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily | |||
:*Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette | |||
:*Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours | |||
:*Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter | |||
:*Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter | |||
====Atelectasis, Due to mucous obstruction==== | |||
* Dosing Information | |||
:*Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily | |||
:*Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette | |||
:*Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours | |||
:*Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter | |||
:*Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter | |||
====Bronchopulmonary disease, acute==== | |||
* Dosing Information | |||
:*Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily | |||
* | :*Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette | ||
* | :*Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours | ||
* | :*Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter | ||
:*Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter | |||
====Complication of surgical procedure - Respiratory complication==== | |||
* Dosing Information | |||
:*Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily | |||
:*Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette | |||
:*Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours | |||
:*Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter | |||
:*Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter | |||
====Cystic fibrosis, Pulmonary complications; Adjunct==== | |||
* Dosing Information | |||
:*Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily | |||
:*Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette | |||
:*Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours | |||
:*Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter | |||
:*Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter | |||
====Diagnostic procedure on lower respiratory tract==== | |||
* Dosing Information | |||
:*Diagnostic bronchial studies: 1 to 2 mL of the 20% solution or 2 to 4 mL of the 10% solution 2 or 3 times prior to the procedure by nebulization or by instillation intratracheally | |||
====Disease of respiratory system, chronic==== | |||
* Dosing Information | |||
:* Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily | |||
2 | :*Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette | ||
:*Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours | |||
:*Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter | |||
:*Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter | |||
====Tracheostomy care==== | |||
* Dosing Information | |||
:*Nebulization into tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily | |||
* | :*Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours | ||
* | :*Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter | ||
* | :*Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter | ||
|offLabelPedGuideSupport=*There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
* | |||
* There | |||
= | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport=* There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications=* Acetylcysteine Injection is contraindicated in patients with previous anaphylactoid reactions to acetylcysteine. | |||
<!--Warnings--> | |||
|warnings='''Anaphylactoid Reactions''' | |||
*Serious anaphylactoid reactions, including death in a patient with asthma, have been reported in patients administered acetylcysteine intravenously. | |||
*Acute [[flushing]] and [[erythema]] of the skin may occur in patients receiving acetylcysteine intravenously. These reactions usually occur 30 to 60 minutes after initiating the infusion and often resolve spontaneously despite continued infusion of acetylcysteine. Anaphylactoid reactions (defined as the occurrence of an acute hypersensitivity reaction during acetylcysteine administration including [[rash]], [[hypotension]], [[wheezing]], and/or [[shortness of breath]]) have been observed in patients receiving intravenous acetylcysteine for acetaminophen overdose and occurred soon after initiation of the infusion.If a reaction to acetylcysteine involves more than simply [[flushing]] and [[erythema]] of the skin, it should be treated as an anaphylactoid reaction. This usually entails administering antihistaminic drugs and in severe cases may require administration of [[epinephrine]]. In addition, the acetylcysteine infusion may be interrupted until treatment of the anaphylactoid symptoms has been initiated and then carefully restarted. If the anaphylactoid reaction returns upon reinitiation of treatment or increases in severity, intravenous acetylcysteine should be discontinued and alternative patient management should be considered. | |||
'''Monitoring patients with asthma''' | |||
*Acetylcysteine Injection should be used with caution in patients with [[asthma]], or where there is a history of bronchospasm. | |||
'''Volume Adjustment: Patients less than 40 kg and Requiring Fluid Restriction''' | |||
*The total volume administered should be adjusted for patients less than 40 kg and for those requiring fluid restriction. To avoid fluid overload, the volume of diluent should be reduced as needed. If volume is not adjusted [[fluid overload]] can occur, potentially resulting in hyponatremia, seizure and death. | |||
* | *For specific treatment information regarding the clinical management of acetaminophen overdose, please contact your regional poison center at 1-800-222-1222, or alternatively, a special health professional assistance line for acetaminophen overdose at 1-800-525-6115. | ||
* | |||
*After proper administration of acetylcysteine, an increased volume of liquified bronchial secretions may occur. When cough is inadequate, the open airway must be maintained by mechanical suction if necessary. When there is a mechanical block due to foreign body or local accumulation, the airway should be cleared by endotracheal aspiration, with or without bronchoscopy. Asthmatics under treatment with acetylcysteine should be watched carefully. Most patients with bronchospasm are quickly relieved by the use of a bronchodilator given by nebulization. If bronchospasm progresses, this medication should be discontinued immediately. | |||
* | |||
<!--Adverse Reactions--> | |||
{{ | <!--Clinical Trials Experience--> | ||
|drugBox={{Drugbox2 | |||
| Verifiedfields = changed | |||
| Watchedfields = changed | |||
| verifiedrevid = 443366456 | |||
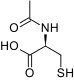
| IUPAC_name = (2''R'')-2-acetamido-3-sulfanylpropanoic acid<ref>{{cite web|title=L-Cysteine, N-acetyl- - Compound Summary|url=http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=581|work=PubChem Compound|publisher=National Center for Biotechnology Information|accessdate=9 January 2012|location=USA|date=25 March 2005|at=Identification}}</ref> | |||
| image = Acetylcysteine2DACS.png | |||
| width = 125 | |||
| image2 = Acetylcysteine3Dan3.gif | |||
| width2 = 200 | |||
| imagename = | |||
| drug_name = | |||
| caption = | |||
{{ | <!-- Clinical data --> | ||
| tradename = Acetadote, Fluimucil, Mucomyst, Parvolex | |||
| Drugs.com = {{drugs.com|monograph|acetylcysteine}} | |||
| MedlinePlus = | |||
| licence_EU = <!-- EMA requires brand name --> | |||
| licence_US = Acetylcysteine | |||
| DailyMedID = 5558a5f5-e821-473b-7d8a-5d33d09f0586 | |||
| pregnancy_AU = B2 | |||
| pregnancy_US = B | |||
| legal_AU = S2 | |||
| legal_CA = <!-- OTC, Rx-only, Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| legal_UK = <!-- GSL, P, POM, CD, CD Lic, CD POM, CD No Reg POM, CD (Benz) POM, CD (Anab) POM or CD Inv POM --> | |||
| legal_US = Rx-only | |||
| routes_of_administration = Oral, injection, inhalation | |||
[[ | <!-- Pharmacokinetic data --> | ||
[[ | | bioavailability = 4-10% (Oral)<ref name = DMO>{{cite web|title=ACETYLCYSTEINE solution [Fresenius Kabi USA, LLC]|work=DailyMed|publisher=Fresenius Kabi USA, LLC|url=http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=75b48fab-cef6-465c-a1e0-d085b3988bc4|date=September 2013|accessdate=8 November 2013}}</ref><ref name = DMI>{{cite web|title=ACETADOTE (acetylcysteine) injection, solution [Cumberland Pharmaceuticals Inc.]|work=DailyMed|publisher=Cumberland Pharmaceuticals Inc.|date=June 2013|accessdate=8 November 2013|url=http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=472f158a-5ab9-4308-8e49-1116e6ea3d39}}</ref><ref name = MD>{{cite book|title=Acetylcysteine|work=Martindale: The Complete Drug Reference|publisher=The Royal Pharmaceutical Society of Great Britain|url=http://www.medicinescomplete.com/mc/martindale/current/ms-3701-r.htm|date=16 November 2012|accessdate=8 November 2013}}</ref><ref name = TGA>{{cite web|title=PRODUCT INFORMATION ACETADOTE® CONCENTRATED INJECTION|work=TGA eBusiness Services|publisher=Phebra Pty Ltd|url=https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-03960-3|format=PDF|date=16 January 2013|accessdate=8 November 2013}}</ref><ref name="pmid3803419">{{cite journal | author = Borgström L, Kågedal B, Paulsen O | title = Pharmacokinetics of N-acetylcysteine in man | journal = Eur. J. Clin. Pharmacol. | volume = 31 | issue = 2 | pages = 217–22 | year = 1986 | pmid = 3803419 | doi = 10.1007/BF00606662 }}</ref> | ||
| protein_bound = 80-83%<ref name = DMO/><ref name = DMI/><ref name = MD/><ref name = TGA/><ref name="pmid3803419"/> | |||
| metabolism = [[Hepatic]]<ref name = DMO/><ref name = DMI/><ref name = MD/><ref name = TGA/><ref name="pmid3803419"/> | |||
| elimination_half-life = 5.6 hours<ref name = DMO/><ref name = DMI/><ref name = MD/><ref name = TGA/><ref name="pmid3803419"/> | |||
| excretion = Renal (22%), faecal (3%)<ref name = DMO/><ref name = DMI/><ref name = MD/><ref name = TGA/><ref name="pmid3803419"/> | |||
<!-- Identifiers --> | |||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 7218-04-4 | |||
| CAS_supplemental = | |||
| ATC_prefix = R05 | |||
| ATC_suffix = CB01 | |||
| ATC_supplemental = {{ATC|S01|XA08}} {{ATC|V03|AB23}} | |||
| PubChem = 581 | |||
| PubChemSubstance = | |||
| IUPHAR_ligand = | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB06151 | |||
| ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| ChemSpiderID = 561 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = WYQ7N0BPYC | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00221 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 28939 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 600 | |||
| synonyms = | |||
<!-- Chemical data --> | |||
| chemical_formula = | |||
| C=5 | H=9 | Ag= | As= | Au= | B= | Bi= | Br= | Cl= | Co= | F= | Fe= | Gd= | I= | |||
| K= | Mn= | N=1 | Na= | O=3 | P= | Pt= | S=1 | Sb= | Se= | Sr= | Tc= | Zn= | charge= | |||
| molecular_weight = 163.195 | |||
| smiles = CC(=O)NC(CS)C(O)=O | |||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChI = 1S/C5H9NO3S/c1-3(7)6-4(2-10)5(8)9/h4,10H,2H2,1H3,(H,6,7)(H,8,9) | |||
| StdInChI_comment = | |||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| StdInChIKey = PWKSKIMOESPYIA-UHFFFAOYSA-N | |||
| density = | |||
| melting_point = 106 | |||
| melting_high = | |||
| melting_notes = | |||
| boiling_point = 108 | |||
| boiling_notes = | |||
| solubility = 200 | |||
| specific_rotation = | |||
| sec_combustion = | |||
}} | |||
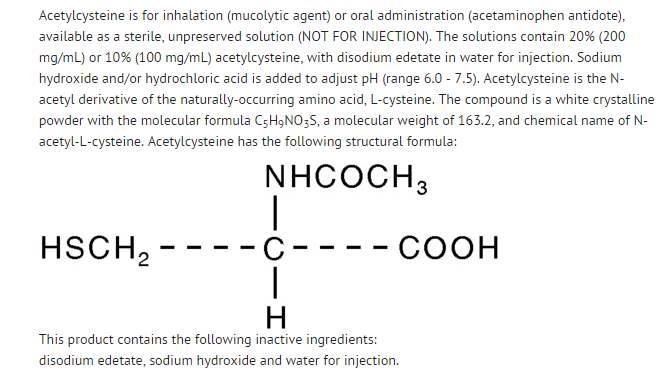
|structure=: [[File:Acetyl cystine structure.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
|packLabel=[[File:ACETYLCYSTEINE0001.jpeg|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
[[File:ACETYLCYSTEINE0002.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
{{ | |alcohol=Alcohol-Acetylcysteine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
|brandNames=* ACETYLCYSTEINE®<ref>{{Cite web | title = ACETYLCYSTEINE- acetylcysteine injection | url =http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=4072b547-c242-4a40-8c91-5481b86cbf1e }}</ref> | |||
}} | |||
[[Category:Drug]] | |||
[[Category:Antioxidants]] | |||
[[Category:Antidotes]] | |||
[[Category:Thiols]] | |||
[[Category:Excipients]] | |||
Latest revision as of 22:41, 14 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Acetylcysteine (injection) is an antioxidant, respiratory system agent that is FDA approved for the treatment of acetaminophen overdose, adjuvant therapy for patients with abnormal, viscid, or inspissated mucous secretions in such conditions as chronic bronchopulmonary disease (chronic emphysema, emphysema with bronchitis, chronic asthmatic bronchitis, tuberculosis, bronchiectasis and primary amyloidosis of the lung), acute bronchopulmonary disease (pneumonia, bronchitis, tracheobronchitis), pulmonary complications of cystic fibrosis, tracheostomy care, pulmonary complications associated with surgery, use during anesthesia, post-traumatic chest conditions, atelectasis due to mucous obstruction, diagnostic bronchial studies (bronchograms, bronchospirometry, and bronchial wedge catheterization). Common adverse reactions include rash, urticaria/facial flushing and pruritus, diarrhea, nausea, vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Acetaminophen overdose
- Acetylcysteine (injection) is an antidote for acetaminophen overdose indicated to prevent or lessen hepatic injury after ingestion of a potentially hepatotoxic quantity of acetaminophen. Overdose incidences are divided into two types; Acute Ingestion or Repeated Supratherapeutic Ingestion (RSI).
- On admission for suspected acetaminophen overdose, a serum blood sample should be drawn at least 4 hours after ingestion to determine the acetaminophen level and will serve as a basis for determining the need for treatment with acetylcysteine. If the patient presents after 4 hours post-ingestion, the serum acetaminophen sample should be determined immediately.
- Acetylcysteine Injection should be administered within 8 hours from acetaminophen ingestion for maximal protection against hepatic injury for patients whose serum acetaminophen levels fall above the "possible" toxicity line on the Rumack-Matthew nomogram (line connecting 150 mcg/mL at 4 hours with 37.5 mcg/mL at 12 hours); [see Acetaminophen Assays – Interpretation and Methodology]. If the time of ingestion is unknown, or the serum acetaminophen level is not available, cannot be interpreted, or is not available within the 8 hour time interval from acetaminophen ingestion, Acetylcysteine Injection should be administered immediately if 24 hours or less have elapsed from the reported time of ingestion of an overdose of acetaminophen, regardless of the quantity reported to have been ingested.
- The aspartate aminotransferase (AST, SGOT), alanine aminotranferase (ALT, SGPT), bilirubin, prothrombin time, creatinine, blood urea nitrogen (BUN), blood glucose, and electrolytes also should be determined in order to monitor hepatic and renal function and electrolyte and fluid balance.
- NOTE: The critical ingestion-treatment interval for maximal protection against severe hepatic injury is between 0 to 8 hours. Efficacy diminishes progressively after 8 hours and treatment initiation between 15 and 24 hours post-ingestion of acetaminophen yields limited efficacy. However, it does not appear to worsen the condition of patients and it should not be withheld, since the reported time of ingestion may not be correct.
Acetaminophen Assays Interpretation and Methodology – Acute Ingestion
- The acute ingestion of acetaminophen in quantities of 150 mg/kg or greater may result in hepatic toxicity. However, the reported history of the quantity of a drug ingested as an overdose is often inaccurate and is not a reliable guide to therapy of the overdose. Therefore, plasma or serum acetaminophen concentrations, determined as early as possible, but no sooner than four hours following an acute overdose, are essential in assessing the potential risk of hepatotoxicity. If an assay for acetaminophen cannot be obtained, it is necessary to assume that the overdose is potentially toxic.
Interpretation of Acetaminophen Assays
- When results of the plasma acetaminophen assay are available, refer to the nomogram in Figure 1 to determine if plasma concentration is in the potentially toxic range. Values above the line connecting 200 mcg/mL at 4 hours with 50 mcg/mL at 12 hours (probable line) are associated with a probability of hepatic toxicity if an antidote is not administered.
- If the predetoxification plasma level is above the line connecting 150 mcg/mL at 4 hours with 37.5 mcg/mL at 12 hours (possible line), continue with maintenance doses of acetylcysteine. It is better to err on the safe side and thus this line, defining possible toxicity, is plotted 25% below the line defining probable toxicity.
- If the predetoxification plasma level is below the line connecting 150 mcg/mL at 4 hours with 37.5 mcg/mL at 12 hours (possible line), there is minimal risk of hepatic toxicity, and Acetylcysteine treatment may be discontinued.
- Estimating Potential for Hepatotoxicity: The following depiction of the Rumack- Matthew nomogram has been developed to estimate the probability that plasma levels in relation to intervals post-ingestion will result in hepatotoxicity.
- The Rumack-Matthew nomogram may underestimate the risk for hepatotoxicity in some patients with risk factors such as chronic alcoholism, malnutrition, or CYP2E1 enzyme inducing drugs (e.g., isoniazid).
Figure 1. Rumack-Matthew Nomogram:
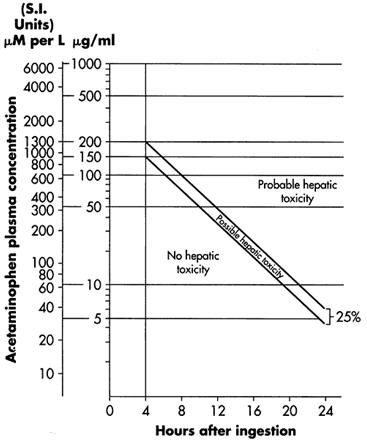
- Figure 1. Michael J Hodgman, Alexander R Garrard, A Review of Acetaminophen Poisoning. Crit Care Clin. 28 (2012) 499-516. Stephen J. Wolf, Kennon Heard, et.al, Clinical Policy: Critical Issues in the Management of Patients Presenting to the Emergency Department with Acetaminophen Overdose. Ann Emerg Med. 2007:50:292-313.
Acetaminophen Assays Interpretation and Methodology – Repeated Supratherapeutic Ingestion
- Repeated Supratherapeutic Ingestion (RSI) is defined as ingestion of acetaminophen at doses higher than those recommended for extended periods of time. The nomogram does not apply to patients with RSI. Treatment is based on the acetaminophen and elevated AST/ALT levels indicative of potential toxicity due to acetaminophen. For specific treatment information regarding the clinical management of repeated supratherapeutic acetaminophen overdose, please contact your regional poison center at 1-800-222-1222, or alternatively, a special health professional assistance line for acetaminophen overdose at 1-800-525-6115.
Figure 2. Acetylcysteine Injection Treatment Flow Chart
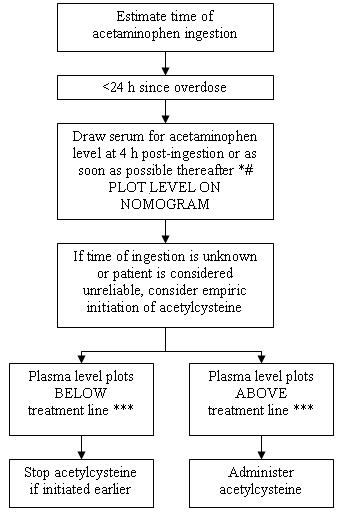
- 1Acetaminophen levels drawn less than 4 hours post-ingestion may be misleading.
- 2With an extended-release preparation, an acetaminophen level drawn less than 8 hours post-ingestion may be misleading. Draw a second level at 4 to 6 hours after the initial level. If either falls above the toxicity line, acetylcysteine treatment should be initiated.
- 3Acetylcysteine may be withheld until acetaminophen assay results are available as long as initiation of treatment is not delayed beyond 8 hours post-ingestion. If more than 8 hours post-ingestion, start acetylcysteine treatment immediately.
Dosing Information
- The total dose of Acetylcysteine Injection is 300 mg/kg given as 3 separate doses and administered over a total of 21 hours. Please refer to the guidelines below for dose preparation based upon patient weight. The total volume administered should be adjusted for patients less than 40 kg and for those requiring fluid restriction (see Tables 1 and 2).
Administration Instructions (Three-Bag Method: Loading, Second and Third Dose)
- Dosing for Patients who weigh 5 kg to 20 kg (Table 1):
- Loading Dose: 150 mg/kg diluted in 3 mL/kg of diluent* administered over 1 hr
- Second Dose: 50 mg/kg diluted in 7 mL/kg of diluent* administered over 4 hrs
- Third Dose: 100 mg/kg diluted in 14 mL/kg of diluent* administered over 16 hrs
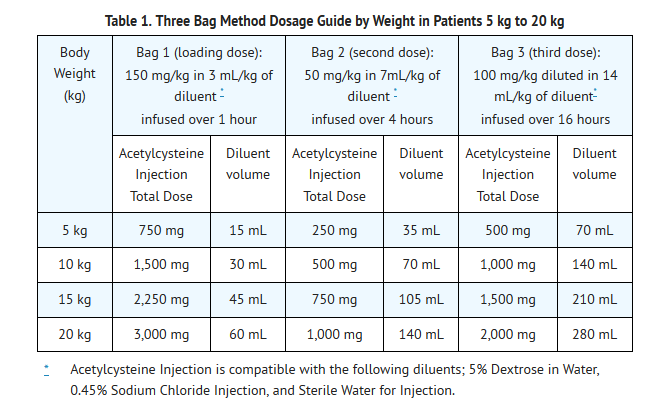
- See also Section 2.2 Volume Adjustment: Patients less than 40 kg and Requiring Fluid Restriction
- Dosing for patients who weigh 21 kg to 40 kg (Table 2):
- Loading Dose: 150 mg/kg diluted in 100 mL of diluent* administered over 1 hr
- Second Dose: 50 mg/kg diluted in 250 mL of diluent* administered over 4 hrs
- Third Dose: : 100 mg/kg diluted in 500 mL of diluent* administered over 16 hrs
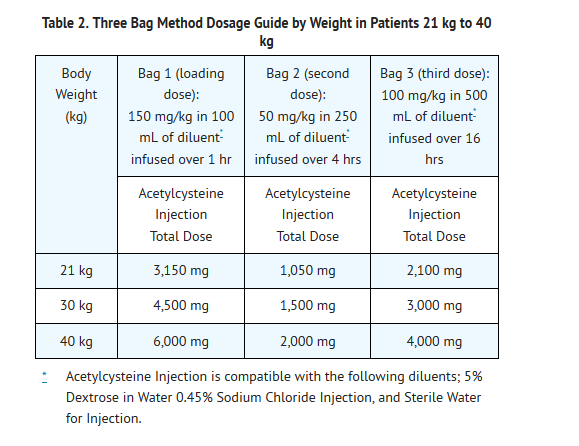
- See also Section 2.2 Volume Adjustment: Patients less than 40 kg and Requiring Fluid Restriction.
- Dosing for patients who weigh 41 kg to 100 kg (Table 3):
- Loading Dose: 150 mg/kg diluted in 200 mL of diluent* administered over 1 hr
- Second Dose: 50 mg/kg diluted in 500 mL of diluent* administered over 4 hrs
- Third Dose: 100 mg/kg diluted in 1,000 mL of diluent administered over 16 hrs
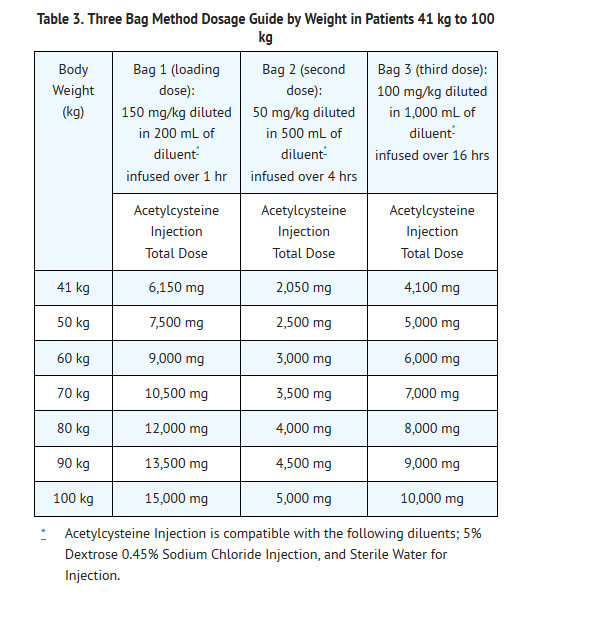
Patients Weighing More Than 100 kg
- No specific studies have been conducted to evaluate the use of or necessity of dosing adjustments in patients weighing over 100 kg. Limited information is available regarding the dosing requirements of patients that weigh more than 100 kg. The dose of Acetylcysteine Injection recommended in these patients should be a loading dose of 15,000 mg infused over a period of one hour followed by a first maintenance dose of 5,000 mg over 4 hours and a second maintenance dose of 10,000 mg over 16 hours (Table 3).
Continued Therapy beyond 21 Hours
- While there is no clinical trial data to support infusions beyond 21 hours there is literature that supports continued infusion of acetylcysteine in some rare instances. In cases of suspected massive overdose, or with concomitant ingestion of other substances, or in patients with preexisting liver disease, the absorption and/or the half-life of acetaminophen may be prolonged, in such cases consideration should be given to the need for continued infusion of N-acetylcysteine beyond 21 hours. Acetaminophen levels and ALT/AST & INR should be checked before the end of the 21-hour infusion. If acetaminophen levels are still detectable, or in cases in which the ALT/AST are still increasing or the INR remains elevated, the infusion should be continued, and the treating physician should contact a US regional poison center at 1-800-222-1222, or alternatively, a “special health professional assistance line for acetaminophen overdose” at 1-800-525-6115 for assistance with dosing recommendations.
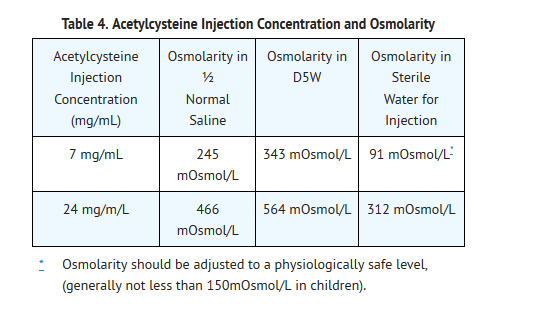
Volume Adjustment: Patients less than 40 kg and Requiring Fluid Restriction
- The total volume administered should be adjusted for patients less than 40 kg and for those requiring fluid restriction. To avoid fluid overload, the volume of diluent should be reduced as clinically needed. If the volume of the infusion is not adjusted, fluid overload can occur, potentially resulting in hyponatremia, seizure and death.
- As Acetylcysteine Injection is hyperosmolar (2600 mOsmol/L), caution is advised when the diluent volume is decreased as the hyperosmolarity of the solution is increased. See Table 4 below for examples.
- Single dose vial, preservative-free, discard unused portion. If vial was previously opened, do not use for intravenous administration.
- Stability studies indicate that the diluted solution is stable for 24 hours at controlled room temperature.
- Note: The color of Acetylcysteine Injection may turn from essentially colorless to a slight pink or purple once the stopper is punctured. The color change does not affect the quality of the product.
Renal Impairment
- No data are available to determine if a dose adjustment in patients with moderate or severe renal impairment is required.
Hepatic Impairment
- Although there was a threefold increase in acetylcysteine plasma concentrations in patients with hepatic cirrhosis, no data are available to determine if a dose adjustment in these patients is required. The published medical literature does not indicate that the dose of acetylcysteine in patients with hepatic impairment should be reduced.
DOSAGE FORMS & STRENGTHS
- Each single dose vial contains 6g/30mL (200 mg/mL) of Acetylcysteine. Acetylcysteine Injection is sterile and can be used for intravenous administration.
Administration of anesthesia for procedure
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Atelectasis, Due to mucous obstruction
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Bronchopulmonary disease, acute
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Complication of surgical procedure - Respiratory complication
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Cystic fibrosis, Pulmonary complications; Adjunct
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Diagnostic procedure on lower respiratory tract
- Dosing Information
- Diagnostic bronchial studies: 1 to 2 mL of the 20% solution or 2 to 4 mL of the 10% solution 2 or 3 times prior to the procedure by nebulization or by instillation intratracheally
Disease of respiratory system, chronic
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Disease of respiratory system, chronic: direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Disease of respiratory system, chronic: percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Disease of respiratory system, chronic: intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Tracheostomy care
- Dosing Information
- Nebulization into tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Acetylcysteine (injection) in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Acetylcysteine (injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Acetaminophen overdose
- Dosing Information
- Oral: initial, 140 mg/kg ORALLY, then 70 mg/kg every 4 hours for 17 doses starting 4 hours after loading dose
- IV: body weight 5 to 20 kg: loading dose, 150 mg/kg in 3 mL/kg of compatible solution (D5W, 0.45%NaCl, or sterile water for injection) IV over 60 minutes, then 50 mg/kg in 7 mL/kg of compatible solution IV over 4 hours, followed by 100 mg/kg in 14 mL/kg of compatible solution IV over 16 hours; may continue beyond 21 hours if clinically indicated; adjust the total volume administered for patients requiring fluid restriction as clinically necessary
- IV: body weight 21 kg to 40 kg: loading dose, 150 mg/kg in 100 mL of compatible solution (D5W, 0.45%NaCl, or sterile water for injection) IV over 60 minutes, then 50 mg/kg in 250 mL of compatible solution IV over 4 hours, followed by 100 mg/kg in 500 mL of compatible solution IV over 16 hours; may continue beyond 21 hours if clinically indicated; adjust the total volume administered for patients less than 40 kg or requiring fluid restriction as clinically necessary
- IV: body weight 41 to 100 kg, loading dose, 150 mg/kg in 200 mL of compatible solution (D5W, 0.45%NaCl, or sterile water for injection) IV over 60 minutes, then 50 mg/kg in 500 mL of compatible solution IV over 4 hours, followed by 100 mg/kg in 1000 mL of solution IV over 16 hours; may continue beyond 21 hours if clinically indicated; adjust the total volume administered for patients requiring fluid restriction as clinically necessary [3]
- IV: body weight over 100 kg, loading dose, 15,000 mg in 200 mL of compatible solution (D5W, 0.45%NaCl, or sterile water for injection) IV over 60 minutes, then 5000 mg in 500 mL of compatible solution IV over 4 hours, followed by 10,000 mg in 1000 mL of solution IV over 16 hours; may continue beyond 21 hours if clinically indicated; adjust the total volume administered for patients requiring fluid restriction as clinically necessary
Administration of anesthesia for procedure
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Atelectasis, Due to mucous obstruction
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Bronchopulmonary disease, acute
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Complication of surgical procedure - Respiratory complication
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Cystic fibrosis, Pulmonary complications; Adjunct
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Diagnostic procedure on lower respiratory tract
- Dosing Information
- Diagnostic bronchial studies: 1 to 2 mL of the 20% solution or 2 to 4 mL of the 10% solution 2 or 3 times prior to the procedure by nebulization or by instillation intratracheally
Disease of respiratory system, chronic
- Dosing Information
- Nebulization into a face mask, mouth piece, or tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Nebulization into a tent or croupette: individualize treatment; use the volume of the 10% or 20% solution that will maintain a very heavy mist in the tent or croupette
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Tracheostomy care
- Dosing Information
- Nebulization into tracheostomy: 1 to 10 mL of the 20% solution or 2 to 20 mL of the 10% solution every 2 to 6 hours; usual dose is 3 to 5 mL of the 20% solution or 6 to 10 mL of the 10% solution 3 to 4 times daily
- Direct instillation into tracheostomy: 1 to 2 mL of a 10% or 20% solution may be given as often as every hour; usual dose is 1 to 2 mL of a 10% or 20% solution every 1 to 4 hours
- Percutaneous intratracheal catheter: 1 to 2 mL of 20% solution or 2 to 4 mL of 10% solution every 1 to 4 hours via a syringe attached to the catheter
- Intratracheal catheter into particular bronchopulmonary tree segment: 2 to 5 mL of the 20% solution via a syringe attached to the catheter
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Acetylcysteine (injection) in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Acetylcysteine (injection) in pediatric patients.
Contraindications
- Acetylcysteine Injection is contraindicated in patients with previous anaphylactoid reactions to acetylcysteine.
Warnings
Anaphylactoid Reactions
- Serious anaphylactoid reactions, including death in a patient with asthma, have been reported in patients administered acetylcysteine intravenously.
- Acute flushing and erythema of the skin may occur in patients receiving acetylcysteine intravenously. These reactions usually occur 30 to 60 minutes after initiating the infusion and often resolve spontaneously despite continued infusion of acetylcysteine. Anaphylactoid reactions (defined as the occurrence of an acute hypersensitivity reaction during acetylcysteine administration including rash, hypotension, wheezing, and/or shortness of breath) have been observed in patients receiving intravenous acetylcysteine for acetaminophen overdose and occurred soon after initiation of the infusion.If a reaction to acetylcysteine involves more than simply flushing and erythema of the skin, it should be treated as an anaphylactoid reaction. This usually entails administering antihistaminic drugs and in severe cases may require administration of epinephrine. In addition, the acetylcysteine infusion may be interrupted until treatment of the anaphylactoid symptoms has been initiated and then carefully restarted. If the anaphylactoid reaction returns upon reinitiation of treatment or increases in severity, intravenous acetylcysteine should be discontinued and alternative patient management should be considered.
Monitoring patients with asthma
- Acetylcysteine Injection should be used with caution in patients with asthma, or where there is a history of bronchospasm.
Volume Adjustment: Patients less than 40 kg and Requiring Fluid Restriction
- The total volume administered should be adjusted for patients less than 40 kg and for those requiring fluid restriction. To avoid fluid overload, the volume of diluent should be reduced as needed. If volume is not adjusted fluid overload can occur, potentially resulting in hyponatremia, seizure and death.
- For specific treatment information regarding the clinical management of acetaminophen overdose, please contact your regional poison center at 1-800-222-1222, or alternatively, a special health professional assistance line for acetaminophen overdose at 1-800-525-6115.
- After proper administration of acetylcysteine, an increased volume of liquified bronchial secretions may occur. When cough is inadequate, the open airway must be maintained by mechanical suction if necessary. When there is a mechanical block due to foreign body or local accumulation, the airway should be cleared by endotracheal aspiration, with or without bronchoscopy. Asthmatics under treatment with acetylcysteine should be watched carefully. Most patients with bronchospasm are quickly relieved by the use of a bronchodilator given by nebulization. If bronchospasm progresses, this medication should be discontinued immediately.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Acetylcysteine (injection) Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Acetylcysteine (injection) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Acetylcysteine (injection) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Acetylcysteine (injection) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Acetylcysteine (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Acetylcysteine (injection) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Acetylcysteine (injection) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Acetylcysteine (injection) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Acetylcysteine (injection) in geriatric settings.
Gender
There is no FDA guidance on the use of Acetylcysteine (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Acetylcysteine (injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Acetylcysteine (injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Acetylcysteine (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Acetylcysteine (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Acetylcysteine (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Acetylcysteine (injection) Administration in the drug label.
Monitoring
There is limited information regarding Acetylcysteine (injection) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Acetylcysteine (injection) and IV administrations.
Overdosage
There is limited information regarding Acetylcysteine (injection) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
There is limited information regarding Acetylcysteine (injection) Mechanism of Action in the drug label.
Structure
Pharmacodynamics
There is limited information regarding Acetylcysteine (injection) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Acetylcysteine (injection) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Acetylcysteine (injection) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Acetylcysteine (injection) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Acetylcysteine (injection) How Supplied in the drug label.
Storage
There is limited information regarding Acetylcysteine (injection) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Acetylcysteine (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

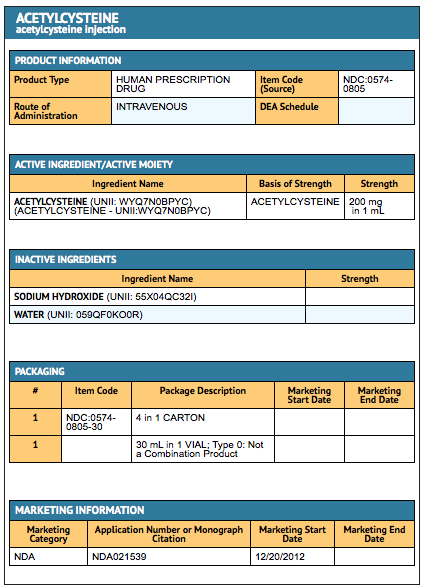
{{#ask: Label Page::Acetylcysteine (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Acetylcysteine (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Acetylcysteine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ACETYLCYSTEINE®[7]
Look-Alike Drug Names
There is limited information regarding Acetylcysteine (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "L-Cysteine, N-acetyl- - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 25 March 2005. Identification. Retrieved 9 January 2012.
- ↑ 2.0 2.1 2.2 2.3 2.4 "ACETYLCYSTEINE solution [Fresenius Kabi USA, LLC]". DailyMed. Fresenius Kabi USA, LLC. September 2013. Retrieved 8 November 2013.
- ↑ 3.0 3.1 3.2 3.3 3.4 "ACETADOTE (acetylcysteine) injection, solution [Cumberland Pharmaceuticals Inc.]". DailyMed. Cumberland Pharmaceuticals Inc. June 2013. Retrieved 8 November 2013.
- ↑ 4.0 4.1 4.2 4.3 4.4 Acetylcysteine. Martindale: The Complete Drug Reference. The Royal Pharmaceutical Society of Great Britain. 16 November 2012. Retrieved 8 November 2013.
- ↑ 5.0 5.1 5.2 5.3 5.4 "PRODUCT INFORMATION ACETADOTE® CONCENTRATED INJECTION" (PDF). TGA eBusiness Services. Phebra Pty Ltd. 16 January 2013. Retrieved 8 November 2013.
- ↑ 6.0 6.1 6.2 6.3 6.4 Borgström L, Kågedal B, Paulsen O (1986). "Pharmacokinetics of N-acetylcysteine in man". Eur. J. Clin. Pharmacol. 31 (2): 217–22. doi:10.1007/BF00606662. PMID 3803419.
- ↑ "ACETYLCYSTEINE- acetylcysteine injection".