Pineal gland tumor
|
Pineal gland tumor Main page |
|
Differentiating features among different various Pineal Gland Tumors |
|---|
|
|
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Fahimeh Shojaei, M.D., Sujit Routray, M.D. [2], Aditya Ganti M.B.B.S. [3]
Synonyms and keywords: Pineal gland tumors; Pineal gland cancer; Pineal gland cancers; Pineal gland neoplasm; Pineal gland neoplasms; Neoplasm of the pineal gland; Neoplasms of the pineal gland; Cancer of the pineal gland; Cancers of the pineal gland
Overview
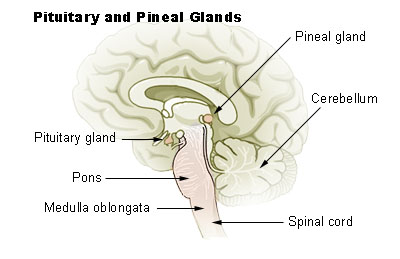
The pineal gland is an endocrine gland that is located in the posterior aspect of the cranial fossa in the brain. The pineal gland is responsible for the secretion of melatonin hormone that regulates the in the circadian cycle sleep and wakefulness. The blood supply of the pineal gland is derived from the posterior cerebral artery from its choroidal branches. The internal cerebral vein drains the blood from the epiphysis cerebri. Histologically the gland consists of cells called pinealocytes. Several different tumors can arise from the pineal gland. Primary pineal cell tumors include pineocytoma, pineoblastoma, and mixed pineal tumors. Tumors that may occur in this region but are not necessarily pineal tumors include germinoma, non-germinoma (eg, teratoma, endodermal sinus tumor, embryonal cell tumor, choriocarcinoma, and mixed tumors), meningioma, astrocytoma, ganglioglioma, and dermoid cysts. Diagnosis of the type of tumor is crucial for treatment. The primary symptom of the tumor would be hydrocephalus. If the pineal gland invades the thalamus, it can cause weakness and loss of sensation in half of the body. Invasion of the hypothalamus would disrupt sleep, impede temperature and water regulation, and cause weight gain. An MRI is important when trying to see the location and size of the tumor. A biopsy is required to determine the type of tumor. Usually, a biopsy is done via a stereotactic or endoscopic procedure. Sometimes biomarkers are used to detect the presence of the tumor, and if these are found in the CSF and blood, then a biopsy might not be needed. Some of these chemicals are beta-human chorionic gonadotropin, carcinoembryonic antigen, and a-fetoprotein.
Classification
Pineal gland tumors are broadly divided into four subcategories. The various types of pineal gland tumors include:[1]
1. Pineal parenchymal tumors: Pineal parenchymal tumors arise directly from the normal functional cells of the pineal gland, pineal parenchymal cells (pineocytes or their precursors), and they are distinct from other pineal gland neoplasms such as astrocytic and germ cell tumors. These tumors are formed after the embryological development of the pineal gland.
2. Pineal germ cell tumors: They are tumors which arise from the embryological abnormalities. They are derived from the germ cells, including sex cells, of the pineal gland during the developmental process of the pineal gland.
3. Astrocytoma of the pineal gland: They arise from the astrocytes, which are a particular kind of star-shaped, glial brain cells around the pineal gland.
4. Pineal metastasis: Pineal metastasis is a cancer that has metastasized to the pineal gland from another location in the body.
Pineal gland tumors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Pathophysiology
Normal Anatomy
- The pineal gland is a small reddish-brown structure that derives its name from its pinecone-like shape.
- The pineal ranges in size from 10 to 14 mm; it is located in the midline, above the tentorium and superior colliculi and below the splenium of the corpus callosum and the vein of Galen, and is attached to the superior aspect of the posterior border of the third ventricle.
- The blood supply of the pineal gland is derived from the posterior cerebral artery from its choroidal branches.
- The internal cerebral vein drains the blood from the epiphysis cerebri.
- Histologically the gland consists of cells called pinealocytes and supporting cells.
Embryology
- Pineal gland develops as a diverticulum in the diencephalic roof of the third ventricle during the second month of gestation.
- The mature gland is suspended from the pineal stalk from the posterior roof of the third ventricle.
- The pineal secretes melatonin, which is involved in diurnal rhythms.
Pathogenesis
- Due to the pineal gland's location, any tumor or cyst formation would lead to the compression of the aqueduct of Sylvius.
- The aqueduct of Sylvius allows the cerebrospinal fluid to circulate out.
- When there is a blockage in aqueduct of Sylvius by an abnormal pineal gland, the passage of the duct is blocked, and CSF pressure builds up, leading to hydrocephalus.
- Increase in intracranial pressure can even be life-threatening, prompting emergency treatment.
- The hydrocephalus can be relieved by the placement of a VP shunt or ventriculostomy.
- Vision changes would also occur due to an involvement of the tectal region.
- The tectal region helps dictate eye movements.
- Fault in the tectal region causes double vision, an issue with focusing on objects, and eye movement impairment.
- The pineal gland can cause Parinaud syndrome due to the increasing size of the gland compressing the pretectal area and superior colliculi of the midbrain.
- Parinaud syndrome prevents a person from moving his or her eyes up and down.
- The thalamus can be affected, and if so, there can be disturbances on that side of the body which would result in weakness and loss of sensation.
- The tumor's effect on the hypothalamus will lead to weight gain, disruption of sleep, disruption of temperature control, and water regulation.
- Cerebellar involvement would result in motor impairment.
- If the tumor of the pineal gland is present in childhood, then endocrine dysfunctions can also result such as precocious pseudopuberty, diabetes insipidus, and a slowed growth rate.
Laboratory Findings
There are no specific laboratory findings associated with pineal gland tumors, however there are certain types of markers associated with diagnosis of germ cell tumors of pineal origin. Germ cell tumors include teratomas, embryonal carcinoma, yolk sac tumor, choriocarcinoma, and the mixed GCTs.
| Type | Oncoprotein | ||
|---|---|---|---|
| AFP | β-HCG | PLAP | |
| Pure Germinoma | - | - | - |
| Mature Teratoma | - | - | |
| Choriocarcinoma | - | + | - |
| Yolk sac tuumor | + | - | - |
| Embryonal carcinoma | - | - | + |
| Mixed GCT | +/- | +/- | +/- |
Differentiating pineal gland tumor from Other Diseases
Pineal gland tumormust be differentiated from other diseases that cause seizure, visual disturbance, and constitutional symptoms. For more information click here.
Differentiating Features Among Various types of Pineal Gland Tumors
| Tumors | Grade | Pathalogic Features | 5-year survival | Cerebrospinal Fluid (CSF) Dissemination | Imaging | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CT | MRI | |||||||||||||
| Pineocytoma |
|
|
|
|
|
| ||||||||
| Pineal choriocarcinoma |
|
|
|
|
| |||||||||
| Pineal embryonal carcinoma |
|
High | ||||||||||||
| Pineal germinoma |
|
|
|
|
|
| ||||||||
| Pineal Parenchymal Tumor of Intermediate Differentiation |
|
|
|
|
|
| ||||||||
| Pineoblastoma |
|
|
|
|
|
| ||||||||
| Papillary Tumor of the Pineal Region |
|
|
|
|
|
| ||||||||
| Germinoma |
|
|
|
|
|
| ||||||||
| Pineal Teratoma |
|
|
|
| ||||||||||
| Pineal Cyst |
|
|
|
|
|
| ||||||||
| Epidermoid and Dermoid Cysts |
|
|
|
|
|
| ||||||||
| Astrocytoma |
|
|
|
|
| |||||||||
| Lipoma |
|
|
|
|
|
| ||||||||
References
- ↑ Pineal region mass. Dr Henry Knipe and Dr Frank Gaillard et al. Radiopaedia 2015. http://radiopaedia.org/articles/pineal-region-mass. Accessed on November 18, 2015
- ↑ Intracranial choriocarcinoma. Frank Gaillard et al. Radiopaedia 2015. http://radiopaedia.org/articles/intracranial-choriocarcinoma. Accessed on December 7, 2015
- ↑ Fujii, Toru; Itakura, Toru; Hayashi, Seiji; Komai, Norihiko; Nakamine, Hirokazu; Saito, Koji (1981). "Primary pineal choriocarcinoma with hemorrhage monitored by computerized tomography". Journal of Neurosurgery. 55 (3): 484–487. doi:10.3171/jns.1981.55.3.0484. ISSN 0022-3085.
- ↑ MRI brain radiographic features of intracranial choriocarcinoma. Frank Gaillard et al. Radiopaedia 2015. http://radiopaedia.org/articles/intracranial-choriocarcinoma. Accessed on December 8, 2015
- ↑ Intracranial embryonal carcinoma. Frank Gaillard et al. Radiopaedia 2015. http://radiopaedia.org/articles/intracranial-embryonal-carcinoma. Accessed on December 4, 2015
- ↑ Histology of germinoma. Wikipedia 2015. https://en.wikipedia.org/wiki/Germinoma. Accessed on December 2, 2015
- ↑ Reddy MP, Saad AF, Doughty KE, Armstrong D, Melguizo-Gavilanes I, Cheek BS; et al. (2015). "Intracranial germinoma". Proc (Bayl Univ Med Cent). 28 (1): 43–5. PMC 4264708. PMID 25552796.
- ↑ CT radiographic features of intracranial germ cell tumors. Dr Ayush Goel and Dr Frank Gaillard et al. Radiopaedia 2015. http://radiopaedia.org/articles/intracranial-germ-cell-tumours. Accessed on December 2, 2015
- ↑ See SJ, Gilbert MR (October 2004). "Anaplastic astrocytoma: diagnosis, prognosis, and management". Semin. Oncol. 31 (5): 618–34. PMID 15497115.
- ↑ Korshunov A, Golanov A, Sycheva R (July 2002). "Immunohistochemical markers for prognosis of anaplastic astrocytomas". J. Neurooncol. 58 (3): 203–15. PMID 12187956.
- ↑ Burger PC, Vogel FS, Green SB, Strike TA (September 1985). "Glioblastoma multiforme and anaplastic astrocytoma. Pathologic criteria and prognostic implications". Cancer. 56 (5): 1106–11. PMID 2990664.