Nedocromil
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Nedocromil is a mast cell stabilizer, ophthalmologic agent that is FDA approved for the treatment of allergic conjunctivitis. Common adverse reactions include headache, ocular burning, irritation and stinging, unpleasant taste, nasal congestion, conjunctivitis, eye redness, photophobia, and rhinitis..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- ALOCRIL® ophthalmic solution is indicated for the treatment of itching associated with allergic conjunctivitis.
Dosing Information
- The recommended dosage is one or two drops in each eye twice a day. ALOCRIL® ophthalmic solution should be used at regular intervals.
- Treatment should be continued throughout the period of exposure (i.e., until the pollen season is over or until exposure to the offending allergen is terminated), even when symptoms are absent.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nedocromil in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nedocromil in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- ALOCRIL® ophthalmic solution is indicated for Allergic conjunctivitis age 3 yr and older.
Dosing Information
- 1 to 2 drops in each eye twice daily
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Nedocromil in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Nedocromil in pediatric patients.
Contraindications
- ALOCRIL® ophthalmic solution is contraindicated in those patients who have shown hypersensitivity to nedocromil sodium or to any of the other ingredients.
Warnings
There is limited information regarding Warnings of Nedocromil in the drug label.
Adverse Reactions
Clinical Trials Experience
- The most frequently reported adverse experience was headache (~40%).
- Ocular burning, irritation and stinging, unpleasant taste, and nasal congestion have been reported to occur in 10 – 30% of patients. Other events occurring between 1 – 10% included asthma, conjunctivitis, eye redness, photophobia, and rhinitis.
- Some of these events were similar to the underlying ocular disease being studied.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Nedocromil in the drug label.
Drug Interactions
There is limited information regarding Drug Interactions of Nedocromil in the drug label.
Use in Specific Populations
Pregnancy
- Reproduction studies performed in mice, rats and rabbits using a subcutaneous dose of 100 mg/kg/day (more than 1600 times the maximum human daily ocular dose on a mg/kg basis) revealed no evidence of teratogenicity or harm to the fetus due to nedocromil sodium. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, ALOCRIL® ophthalmic solution should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Nedocromil in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Nedocromil during labor and delivery.
Nursing Mothers
- After intravenous administration to lactating rats, nedocromil was excreted in milk. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ALOCRIL® ophthalmic solution is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in children below the age of 3 years have not been established.
Geriatic Use
- No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Gender
There is no FDA guidance on the use of Nedocromil with respect to specific gender populations.
Race
There is no FDA guidance on the use of Nedocromil with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Nedocromil in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Nedocromil in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Nedocromil in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Nedocromil in patients who are immunocompromised.
Administration and Monitoring
Administration
- Ophthalmic solution
Monitoring
There is limited information regarding Monitoring of Nedocromil in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Nedocromil in the drug label.
Overdosage
There is limited information regarding Chronic Overdose of Nedocromil in the drug label.
Pharmacology
| |
Nedocromil
| |
| Systematic (IUPAC) name | |
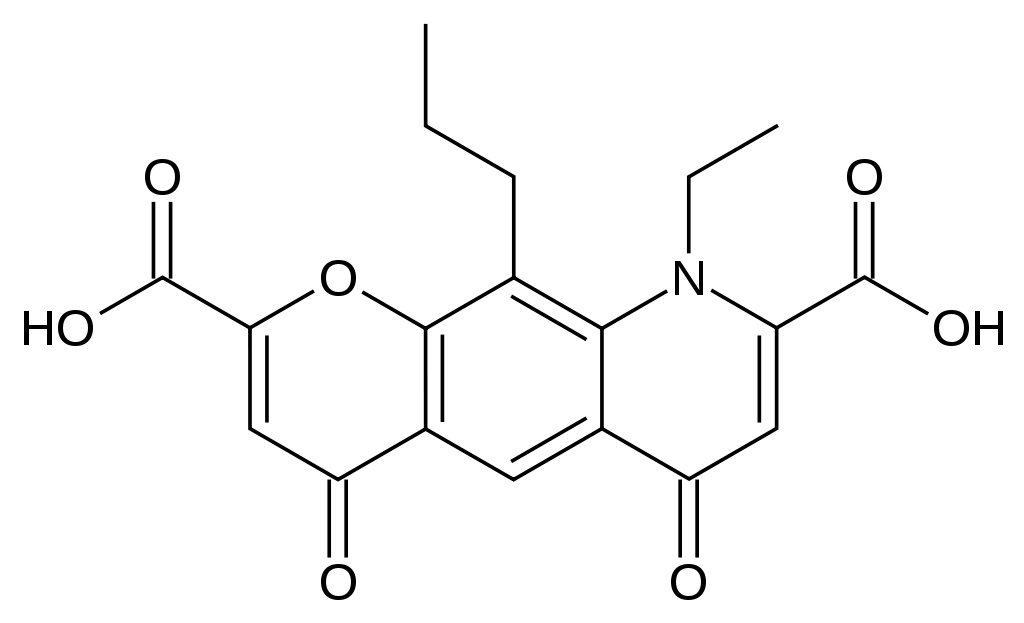
| 9-ethyl-4,6-dioxo-10-propyl-6,9-dihydro-4H-pyrano[3,2-g]quinoline-2,8-dicarboxylic acid | |
| Identifiers | |
| CAS number | |
| ATC code | R01 R03BC03 (WHO), S01GX04 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 371.341 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 89% |
| Metabolism | not metabolized |
| Half life | ~3.3 hours |
| Excretion | excreted unchanged |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Inhalation and eye drops |
Mechanism of Action
- Nedocromil sodium is a mast cell stabilizer. Nedocromil sodium inhibits the release of mediators from cells involved in hypersensitivity reactions. Decreased chemotaxis and decreased activation of eosinophils have also been demonstrated.
- In vitro studies with adult human bronchoalveolar cells showed that nedocromil sodium inhibits histamine release from a population of mast cells having been defined as belonging to the mucosal sub type and inhibits beta-glucuronidase release from macrophages.
Structure
- ALOCRIL® (nedocromil sodium ophthalmic solution) 2% is a clear, yellow, sterile solution for topical ophthalmic use.
- Nedocromil sodium is represented by the following structural formula:

Pharmacodynamics
There is limited information regarding Pharmacodynamics of Nedocromil in the drug label.
Pharmacokinetics
- Nedocromil sodium exhibits low systemic absorption. When administered as a 2% ophthalmic solution in adult human volunteers, less than 4% of the total dose was systemically absorbed following multiple dosing. Absorption is mainly through the nasolacrimal duct rather than through the conjunctiva. It is not metabolized and is eliminated primarily unchanged in urine (70%) and feces (30%).
Nonclinical Toxicology
- A two-year inhalation carcinogenicity study of nedocromil sodium at a dose of 24 mg/kg/day (approximately 400 times the maximum recommended human daily ocular dose on a mg/kg basis) in Wistar rats showed no carcinogenic potential.
- Nedocromil sodium showed no mutagenic potential in the Ames Salmonella/microsome plate assay, mitotic gene conversion in Saccharomyces cerevisiae, mouse lymphoma forward mutation and mouse micronucleus assays.
- Reproduction and fertility studies in mice and rats showed no effects on male and female fertility at a subcutaneous dose of 100 mg/kg/day (more than 1600 times the maximum recommended human daily ocular dose).
Clinical Studies
There is limited information regarding Clinical Studies of Nedocromil in the drug label.
How Supplied
ALOCRIL® (nedocromil sodium ophthalmic solution) 2% is supplied sterile in opaque white LDPE plastic bottles with dropper tips and white high impact polystyrene (HIPS) caps as follows:
5 mL in 10 mL bottle NDC 0023-8842-05
Rx only
Revised: 12/2012
© 2013 Allergan, Inc. Irvine, CA 92612, U.S.A. ® marks owned by Allergan, Inc. Made in the U.S.A.
71761US12
Storage
- Storage: Store at 2º–25º C (36º–77º F).
Images
Drug Images
{{#ask: Page Name::Nedocromil |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL
NDC 0023-8842-05
Alocril® (nedocromil sodium
ophthalmic solution) 2%
STERILE
For topical application in the eye
5 mL
ALLERGAN

{{#ask: Label Page::Nedocromil |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be advised to follow the patient instructions listed on the Information for Patients sheet.
- Users of contact lenses should refrain from wearing lenses while exhibiting the signs and symptoms of allergic conjunctivitis.
- Patients should be instructed to avoid allowing the tip of the dispensing container to contact the eye, surrounding structures, fingers, or any other surface in order to avoid contamination of the solution by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.



Precautions with Alcohol
- Alcohol-Nedocromil interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Alocril®
Look-Alike Drug Names
There is limited information regarding Nedocromil Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Nedocromil
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Nedocromil |Label Name=Nedocromil11.png
}}
{{#subobject:
|Label Page=Nedocromil |Label Name=Nedocromil11.png
}}