Cardiac output
(Redirected from Low output state)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Assistant Editor(s)-in-Chief: Rim Halaby
Overview
- Cardiac output (CO) is defined as the amount of blood pumped by the left ventricle in unit time.
- CO = Stroke Volume x Heart Rate
- Therefore, if there are 70 beats per minute, and 70 ml blood is ejected with each beat of the heart, the cardiac output is 4900 ml/minute.
- The normal cardiac output is 5-6L/min and it can increase up to 5 times during exercise.
- CO is an indicator of the left ventricular function.
- CO = Stroke Volume x Heart Rate
- Cardiac index (CI) is the output of the heart per minute per body surface area.
- CI = CO / Body Surface Area
- The normal cardiac index is 3.2 L/min/m2.[1]
- Stroke volume (SV) is the amount of blood pumped by the left ventricle in a single cardiac cycle.
- Ejection Fraction EF is the fraction of blood ejected by the left ventricle (LV) during the contraction phase of the cardiac cycle (known as systole).
- EF= (Stroke Volume / End Diastolic Volume) x 100
Factors Affecting the Cardiac Output
- CO = Stroke volume x Heart rate
- The cardiac output changes when there is any change in the stroke volume, the heart rate or both.
Factors Affecting the Heart Rate:
- The electrical activity of the heart is generated spontaneously in the sinoatrial node. However, the autonomic nervous system affects the speed at which the electrical activity of the heart is generated and hence affects the heart rate.
- The sympathetic nervous system increases the heart rate (positive chronotropy).
- The parasympathetic nervous system decreases the heart rate (negative chronotropy).[2]
Factors Affecting the Stroke Volume:
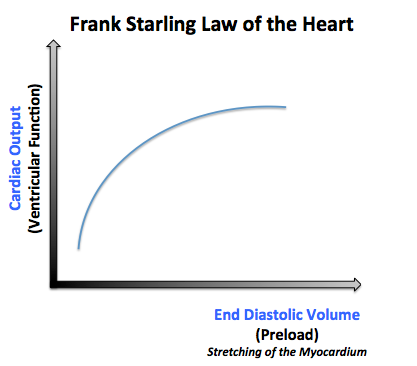
- 1- Preload:
- 2- Afterload:
- The afterload is the pressure corresponding to the mean arterial pressure that the heart needs to overcome when pumping blood.
- When the afterload increases, it makes it harder for the heart to pump the blood, and thus the volume remaining in the ventricles after ventricular contraction (end systolic volume) will increase and the stroke volume will be low.
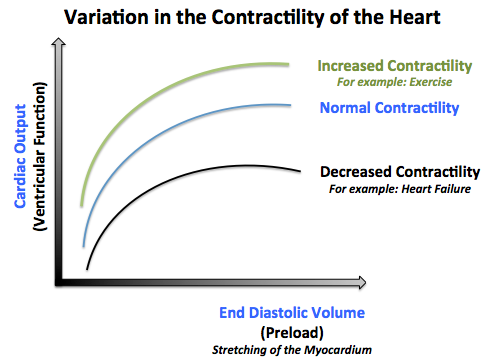
- 3- The contractility of the heart:
- The contractility of the heart is defined as the intrinsic force with which the heart contracts.
- Factors that increase the contractility of the heart (positive ionotropy) are: catecholamines, xanthines (caffeine), medications (Digitalis).
- Factors that decrease the contractlity of the heart (negative ionotropy) are: hypercapnea, hypoxia, acidosis, medications (quinidine, procainamide, barbiturates), heart failure.[3]
Variation of Cardiac Output with Exercise
When cardiac output increases in a healthy but untrained individual, most of the increase can be attributed to increase in heart rate. Change of posture, increased sympathetic nervous system activity, and decreased parasympathetic nervous system activity can also increase cardiac output. Heart rate can vary by a factor of approximately 3, between 60 and 180 beats per minute, whilst stroke volume can vary between 70 and 120 ml, a factor of only 1.7.
Clinical Correlation
- Diseases of the cardiovascular system are often associated with changes in CO.
- Cardiomyopathy and heart failure cause a reduction in cardiac output.
- Hypertension, infection and sepsis are known to increase cardiac output.
Regional Distribution of the Cardiac Output
| Name of circulation | % of cardiac output | Autoregulation | Perfusion | Comments |
| pulmonary circulation | 100% (deoxygenated) | Vasoconstriction in response to hypoxia | ||
| cerebral circulation | 15%[4] | high | under-perfused | Fixed volume means intolerance of high pressure. Minimal ability to use anaerobic respiration |
| coronary circulation | 5% | high | under-perfused | Minimal ability to use anaerobic respiration. Blood flow through the left coronary artery is at a maximum during diastole (in contrast to the rest of systemic circulation, which has a maximum blood flow during systole.) |
| splanchnic circulation | 15% | low | Flow increases during digestion. | |
| hepatic circulation | 15% | Part of portal venous system, so oncotic pressure is very low | ||
| renal circulation | 25% | high | over-perfused | Maintains glomerular filtration rate |
| skeletal muscle circulation | 17%[5] | Perfusion increases dramatically during exercise. | ||
| cutaneous circulation | 2%[6] | over-perfused | Crucial in thermoregulation. Significant ability to use anaerobic respiration |
Measuring Cardiac Output
Non Invasive Measurement of the Cardiac Output
- A non-invasive method, often used in teaching students of physiology, reasons as follows:
- The pressure in the heart rises as blood is forced into the aorta.
- The more stretched the aorta, the greater the pulse pressure which is the difference between the systolic and diastolic blood pressures.
- In healthy young subjects, each additional 2 ml of blood results in a 1 mmHg rise in pressure.
- Therefore:
- Stroke Volume = 2 ml × Pulse Pressure
- Cardiac Output = 2 ml × Pulse Pressure × Heart Rate
Magnetic Resonance Imaging
- Velocity encoded phase contrast Magnetic Resonance Imaging (MRI)[7] is the most accurate technique for measuring flow in large vessels in mammals. [8]
- Velocity encoded MRI is based on detection of changes in the phase of proton precession. These changes are proportional to the velocity of the movement of those protons through a magnetic field with a known gradient. When using velocity encoded MRI, the result of the MRI scan is two sets of images for each time point in the cardiac cycle.
- One is an anatomical image.
- The other is an image where the signal intensity in each pixel is directly proportional to the through-plane velocity.
- The average velocity in a vessel, i.e. the aorta or the pulmonary artery, is hence quantified by measuring the average signal intensity of the pixels in the cross section of the vessel, and then multiplying by a known constant.
- The flow is calculated by multiplying the mean velocity by the cross-sectional area of the vessel.
- This flow data can be used to graph flow versus time.
- The area under the flow versus time curve for one cardiac cycle is the stroke volume.
- The length of the cardiac cycle is known and determines heart rate
- Cardiac output can be calculated as the product of stroke volume and heart rate.
The Fick Principle
- The Fick principle involves measuring:
- VO2 consumption per minute using a spirometer (with the subject re-breathing air) and a CO2 absorber
- The oxygen content of blood taken from the pulmonary artery (representing mixed venous blood)
- The oxygen content of blood from a cannula in a peripheral artery (representing arterial blood)
- From these values, we know that: VO2= (CO x CA) - ( CO x CV)
where CO = Cardiac Output; CA = Oxygen concentration of arterial blood and CV = Oxygen concentration of venous blood.
- This allows us to say: CO = 100 x (CA- CV) / VO2
Dilution methods
- This method measures how fast flowing blood can dilute an indicator substance introduced to the circulatory system, usually using a pulmonary artery catheter.
- Early methods used a dye, the cardiac output being inversely proportional to the concentration of dye sampled downstream. More specifically, the cardiac output is equal to the quantity of indicator dye injected divided by the area under the dilution curve measured downstream (the Stewart-Hamilton equation).
Doppler method
- This technique uses ultrasound and the Doppler effect to measure cardiac output.
- The blood velocity through the aorta cause a 'Doppler shift' in the frequency of the returning ultrasound waves.
- Echocardiographic measurement of the aortic root cross-sectional area (or, alternatively, the descending aorta area) multiplied by the measured velocity time integral of flow through that area and heart rate, yields the cardiac output.
Pulmonary Artery Thermodilution (Trans-right-heart Thermodilution)
- The pulmonary artery catheter (PAC) also known as the Swan-Ganz thermodilution catheter provides right heart blood pressures.
- Using the PAC thermodilution cardiac output can be measured.
- Modern catheters are fitted with a distal heated filament, which allows automatic thermodilution measurement via heating the blood and measuring the resultant thermodilution trace.
- This provides near continuous cardiac output monitoring. The PAC is used in assessment of hemodynamic status and direct intracardiac and pulmonary artery pressures. The distal (pulmonary artery) port allows sampling of mixed venous blood for the assessment of oxygen transport and the calculation of derived parameters such as oxygen consumption, oxygen utilization coefficient, and intrapulmonary shunt fraction.
- The PAC is balloon tipped which can be inflated to occlude the pulmonary artery, the subsequence back pressure is a reflection of the left atrial filling pressure and until recently was considered a good indicator of preload.
- The pulmonary artery wedge pressure (PAWP) has been superseded by more reliable techniques such as intrathoracic blood volume or stroke volume variation as indicators of volume status. The PAC also allows sampling of mixed venous blood, the oxygen content of which can be used to indicate the adequacy of overall oxygen delivery. The PAC has fallen out of common use as clinicians favour less invasive, less hazardous technologies for monitoring haemodynamic status. Considerable controversy exists over whether the PAC increases mortality; recent studies suggest it neither increases nor improves mortality. Complications such as cardiac tamponade, pulmonary artery rupture and air emboli are a danger.
PulseCO and PiCCO Technology
- PiCCO (PULSION Medical Systems AG, Munich, Germany) and PulseCO (LiDCO Ltd, London, England) generate continuous cardiac output by analysis of the arterial blood pressure waveform.
- In both cases, an independent technique is required to provide initial calibration of the continuous cardiac output analysis, as arterial waveform analysis cannot account for unmeasured variables such as compliance of the vascular tree.
- In the case of PiCCO, transpulmonary thermodilution is used as the independent technique. This uses the Stewart-Hamilton principle outlined above, but measured from central venous line to a central (i.e. femoral or axillary) arterial line. The cardiac output derived from this cold-saline thermodilution is used to calibrate the arterial pulse contour analysis, which can then provide continuous cardiac output monitoring. The PiCCO algorithm is dependent on blood pressure waveform morphology (i.e. mathematical analysis of the pulse contour waveform) and calculates continuous cardiac output as described by Wesseling and co-workers. Transpulmonary thermodilution spans right heart, pulmonary circulation and left heart; this allows further mathematical analysis of the thermodilution curve, giving measurements of cardiac filling volumes (GEDV), intrathoracic blood volume, and extravascular lung water.
- In the case of LiDCO, the independent calibration technique is lithium dilution, again using the Stewart-Hamilton principle. Lithium dilution has the advantage of being usable from a peripheral vein to a peripheral arterial line; however, it does not provide information on cardiac filling volumes and extravascular lung water. Dilution measurements cannot be performed too frequently, and can be subject to error in the presence of certain muscle relaxants. The PulseCO algorithm used by LiDCO is based on pulse power derivation and is not dependent on waveform morphology.
FloTrac technology
- A more recent development is the FloTrac system which can derive cardiac output from the arterial waveform without the need for an independent method of calibration.
- Hence continuous cardiac output can be measured directly from a conventional arterial line.
- This method has yet to be extensively evaluated, but early studies suggest that it is accurate. Another similar system that uses the arterial pulse is the pressure recording analytical method (PRAM).
Impedance cardiography
- Impedance cardiography (ICG) is an advanced technique which was developed by NASA. It calculates cardiac output based on the measurement of changes in impedance in the chest over the cardiac cycle.
- This technique has progressed clinically (often called BioZ, i.e. biologic impedance, as promoted by the leading manufacturer in the US) and allows low cost, non-invasive estimations of cardiac output and total peripheral resistance, using only 4 paired skin electrodes, with minimal removal of clothing in physician offices having the needed equipment.
External links
References
- ↑ Barrett KE, Barman SM, Boitano S, Brooks HL. Chapter 30. The Heart as a Pump. In: Barrett KE, Barman SM, Boitano S, Brooks HL, eds. Ganong's Review of Medical Physiology. 24th ed. New York: McGraw-Hill; 2012.
- ↑ Barrett KE, Barman SM, Boitano S, Brooks HL. Chapter 30. The Heart as a Pump. In: Barrett KE, Barman SM, Boitano S, Brooks HL, eds. Ganong's Review of Medical Physiology. 24th ed. New York: McGraw-Hill; 2012.
- ↑ Barrett KE, Barman SM, Boitano S, Brooks HL. Chapter 30. The Heart as a Pump. In: Barrett KE, Barman SM, Boitano S, Brooks HL, eds. Ganong's Review of Medical Physiology. 24th ed. New York: McGraw-Hill; 2012.
- ↑ Essentials of Human Physiology by Thomas M. Nosek. Section 3/3ch11/s3c11_13.
- ↑ Essentials of Human Physiology by Thomas M. Nosek. Section 3/3ch11/s3c11_2.
- ↑ Essentials of Human Physiology by Thomas M. Nosek. Section 3/3ch11/s3c11_10.
- ↑ Arheden H, Stahlberg F. Blood flow measurements. In: MRI and CT of the Cardiovascular System, 2nd edition, Editors: Higgins CB & de Roos A 2006:71-90.
- ↑ Arheden H, et al, Radiology, 1999.