Congenital pulmonary anomalies
| Congenital pulmonary anomalies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Developmental Anatomy
Intrauterine stage
- Four periods of development:
- embryonic
- pseudoglandular
- canalicular
- terminal sac
- Embryonic period begins at 26 days with ventral protrusion of the foregut and ends at 32 days with appearance of 5 lobar bronchi
- Pseudoglandular period lasts from 5th to 16th weeks and is characterized by rapid branching and formation of all conducting airways
- Canalicular period lasts from 16th to 25th weeks and is characterized by capillary ingrowth and appearance of saccules
- Terminal sac or alveolar period begins at 25 weeks, and alveolar development begins between the 30th and 36th weeks
- Type I and II epithelial differentation typically occurs at 28 weeks
Neonatal stage
- Precursors of typical acinar unit are present at birth: bronchioles, transitional ducts, and terminal saccule
- Alveolar development continues after birth with remodeling and multiplication
- The total adult number of alveoli are not reached until at least age 8
- Alveolar enlargement continues until adulthood, although no new alveoli are added
Histology
- The mature lung is characterized by closely packed alveoli divided by thin septa, occupied by capillaries
- Capillary endothelium is typically a single cell layer with few organelles and thin cytoplasmic matrix
- Over 95% of alveolar epithelium is type I cells, which are also very thin with few organelles
- Type II alveolar cells are cuboidal and secrete surfactant
- Surfactant synthesis peaks at term and decreases to adult levels shortly thereafter
- An increase in the lecithin-sphingomyelin ratio to more than 2:1 occurs just before birth
- Type II cells can be stimulated to produce this pattern of phospholipid by steroids, thyroxine, estrogens, beta-agonists, and increases in ventilation or tidal volume
- Surfactant stabilizes the alveoli, lowers surface tension to keep alveoli open at low volumes, prevents alveolar wall adhesion, and helps maintain pulmonary compliance
Anatomic Variants with Normal Parenchyma
- Superior segment of lower lobe delineated by separate fissure
- Medial accessory left lower lobe
- Azygous lobe - mesentery of azygous vein forms double fold of visceral pleura that isolates part of right upper lobe
- Bilateral bilobation or trilobation
- Situs inversus thoracis or totalis - totalis form associated with Kartagener's syndrome (situs inversus, bronchiectasis, pansinusitis - immotile cilia syndrome)
Agenesis, Aplasia, and Hypoplasia
Agenesis and Aplasia
- Agenesis is the complete absence of carina, main bronchus, lung, and pulmonary vasculature
- Aplasia is the development of carina and rudimentary main bronchus; absence of lung and pulmonary vessels
- Bilateral pulmonary agenesis extremely rare and uniformly fatal
- More than 50% of patients with unilateral agenesis have other associated anomalies
- Agenesis does not show a right or left predominance
- Lobar agenesis is less common that total lung agenesis
Hypoplasia
- Failure to obtain adequate size; all components are present, but incompletely developed
- Severity of hypoplasia determines the degree of respiratory compromise
- Major abnormality is a decrease in the number of airway generations and pulmonary artery branchings
- Two general causes: large diaphragmatic hernia or primary embryologic defect
- Hernia interferes with alveolar development during last 2 months of intrauterine growth
- Embryologic defects occur early and may be associated with other syndromes
- Associated anomalies include oligohydramnios, prune-belly syndrome, Potter's syndrome, and scimitar syndrome
- If associated with diaphragmatic hernia, the hypoplastic lung should not be removed at surgery, as it will recover some function with time
Congenital Lobar Emphysema
Congenital lobar emphysema is over-expansion of a pulmonary lobe with compression of other lobes and shifting of mediastinum.
Pathogenesis
- Accounts for about 50% of all congenital lung malformations
- Intrinsic causes: bronchomalacia due to abnormal cartilaginous support, mucus plugging, bronchial torsion, redundant mucosa, foreign body aspiration
- Extrinsic causes: bronchial compression from pulmonary artery sling, great vessel aneurysms, enlarged cardiac chambers
- Pulmonary hypoplasia: decreased number of alveoli become overdistended due to air trapping
- Alveolar hyperplasia: excessive number of alveoli overexpanded a polyalveolar lobe
- Most common in LUL, then RUL, then RML
Symptoms
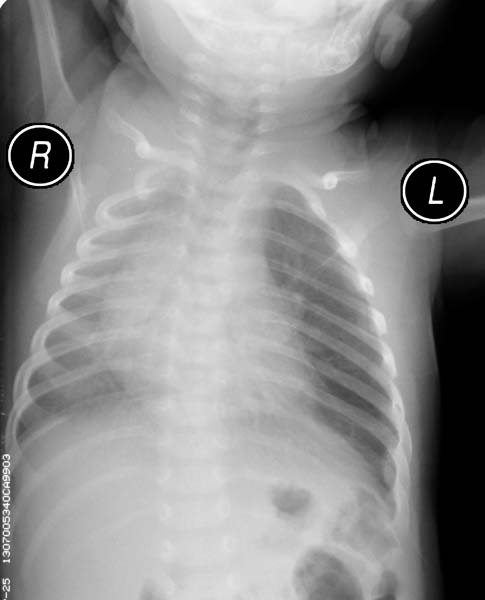
- Respiratory distress occurs in 50% of patients in the first week of life (50%)
- 80% of infants will be symptomatic by 6 months of age
- Most common symptoms are tachypnea, cough, cyanosis, and dyspnea
- Pulmonary infection in the affected lobe occurs most commonly between 1 and 6 months of age
- Presentation is uncommon after age 6 months
- Physical findings include hyperresonance and decreased breath sounds on the affected side
- CXR. shows hyperlucency on the affected side and mediastinal shift to the opposite side
- Air bronchography demonstrates bronchial wall collapse with expiration if bronchomalacia is the cause
Diagnosis
The imaging findings are
- If the plain radiograph is obtained during the neonatal period, the emphysematous lobe may be opaque and homogeneous because of fetal lung fluid or it may show a diffuse reticular pattern that represents distended lymphatic channels filled with fetal lung fluid.
- As the fluid is absorbed, the affected segment or lobe becomes hyperlucent, progresing from alveolar opacification to interstitial reticulation to general hyperlucency.
- Adjacent lobes and structures may be compressed by the emphysematous lobe, and sometimes ipsilateral and contralateral atelectasis may occur.
Patient #1
Patient#2
Treatment
- Indication for surgery is life-threatening progressive insufficiency from normal lung compression
- Careful induction of anesthesia and positive pressure ventilation
- Immediate thoracotomy necessary in the critically ill infant, as ventilation may worsen normal lung compression by rapidly expanding the affected lung
- Surrounding atelectatic normal lung tissue should be preserved
- Lobectomy should result in cure
- Long-term outlook is good with relatively normal PFT's
Pulmonary Cysts
Pulmonary Cysts are cystic structures within the pulmonary parenchyma. The composition of the cyst wall is determined by its origin: bronchial glands, cartilage, or alveolar epithelium.
Pathology
- Congenital cysts are typically unilocular and confined to a single lobe; the lower lobes are more commonly involved
- Congenital cysts that persist for more than 1 year are unlikely to resolve spontaneously
- Multiple cysts are rarely congenital and are probably acquired; causes include staphylococcal pneumonia and cystic fibrosis.
- Multiple cysts may fluctuate in size and can develop rapidly
- CXR usually adequate for identification
Symptoms
- Expansion results in respiratory distress
- Infection causes fever, cough, and sepsis
Treatment
- Solitary congenital cysts can be treated with cystectomy or lobectomy if necessary
- Infected solitary cysts should be treated with antibiotics and resected when quiescent
- Multiple cysts should be treated as part of underlying systemic disease; surgical intervention is rarely required and is contraindicated for pneumatoceles
- Chest tube placement or aspiration for diagnosis is discouraged for tension cysts, as this can result in empyema
Cystic Adenomatoid Malformation
Cystic adenomatoid malformation is thoracic hamartoma with overgrowth in varying amounts of bronchioles and alveoli, so it characteristically can range from cystic to nearly solid masses within the lung. Cartilage, however, is not present in this lesion.
Pathology
- Typically confined to a single lobe
- Progressive air trapping in cystic areas leads to over distension of involved lobe
- Three subtypes (see table below)
- High incidence of associated congenital anomalies and death with type II

Symptoms
- Progressive respiratory distress in a newborn infant
- Tachypnea, subcostal retraction, cyanosis
- Older child - chronic pulmonary infection
- Differential should include congenital diaphragmatic hernia, and barium swallow will differentiate the two
Treatment
- Identification of the lesion is the indication for surgery, with or without symptoms
- Lobectomy is usually adequate
- Attempt at segmentectomy is usually met with prolonged air leak and complications
Bronchogenic Cysts
Bronchogenic cysts are congenital cystic lesions arising from abnormal budding of primitive tracheobronchial tree; composed of fibrous tissue interposed with normal bronchial elements
Pathology
- Cysts can occur either in the parenchyma (70%) or the mediastinum (30%)
- The most common locations are paratracheal, carinal, hilar, and paraesophageal
- Generally round and unilocular
- Lined with ciliated columnar epithelium
- Most do not communicate with the tracheobronchial tree
- Can mimic lobar emphysema from bronchial obstruction
- Can become secondarily infected
Symptoms
- Most common presentation in the neonate is progressive dyspnea, wheezing, stridor, and cyanosis
- Obstructive symptoms occur most commonly in infants less than 1 year of age
- Paratracheal, carinal, and hilar cysts are commonly asymptomatic
- Communicating cysts are almost always symptomatic: productive cough, fever, hemoptysis
- CXR shows homogenous, sharply delineated cyst; air/fluid levels may be present in communicating cyst
- CT is the test of choice for diagnosis and anatomic delineation
Treatment
- Indications for surgery include increasing cyst size, air/fluid level, symptomatic cyst, and subcarinal cyst
- Small, asymptomatic cysts can be followed with interval CXR
- Cyst excision with sparing of surrounding pulmonary tissue is the treatment of choice
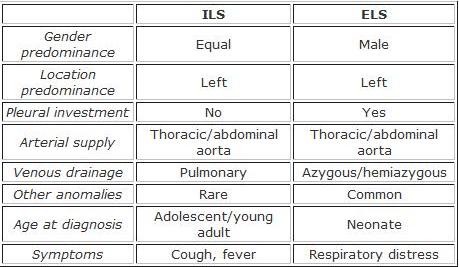
Intralobar Pulmonary Sequestration
Intralobar pulmonary sequestration is a segment of lung parenchyma that is within the normal pleural confines, but does not communicate with the tracheobronchial tree and is supplied by the systemic circulation.
Pathology
- Unicystic or polycystic parenchyma with extensive fibrosis and vascular sclerosis
- Arterial supply is usually from the thoracic aorta (75%) or abdominal aorta (20%)
- Venous drainage is usually to the pulmonary veins
- Right-sided lesions more often have other venous drainage
- Much more frequent than extralobar type
Symptoms
- Recurrent episodes of infection in older children and adolescents
- CXR shows cystic structure with or without air-fluid level
- CT is procedure of choice; aortography and barium swallow may be necessary for anatomy
Treatment
- Identification of the lesion is indication for surgery
- Careful ligation of the anomalous artery followed by resection
- Prevents long-term infection and possible neoplastic changes
Extraobar Pulmonary Sequestration
Extraobar pulmonary sequestration is a segment of lung parenchyma with distinct and separate pleural investment; does not communicate with the tracheobronchial tree and is supplied by the systemic circulation.
Pathology
- Mass of loose, spongy parenchyma with multiple small cysts and dilated bronchioles and ducts
- Arterial supply is also usually from the thoracic or abdominal aorta
- Venous drainage is usually to the azygous or hemiazygous system
- More common on the left side and usually found between the lower lobe and the diaphragm
Symptoms
- Most present in neonatal period due to respiratory distress
- CXR shows triangular homogenous mass with apex pointing toward hilum
- CT may provide additional localization, but vessels often too small to identify
Treatment
- Identification of the lesion is indication for surgery
- Careful ligation of the anomalous artery followed by resection
- As with intralobar sequestration, resection prevents long-term infection and possible neoplastic changes
Source
- CTSNet Wiki Notes
References
- Olutoye OO, Coleman BG, Hubbard AM, Adzick NS. Prenatal diagnosis and management of congenital lobar emphysema. J Pediatr Surg. 2000 May;35(5):792-5.
- Evrard V, Ceulemans J, Coosemans W, De Baere T, De Leyn P, Deneffe G, Devlieger H, De Boeck C, Van Raemdonck D, Lerut T. Congenital parenchymatous malformations of the lung. World J Surg. 1999 Nov;23(11):1123-32.
- Cloutier MM, Schaeffer DA, Hight D. Congenital cystic adenomatoid malformation. Chest 1993 103(3):761-4.
- Martinod E, Pons F, Azorin J, Mouroux J, Dahan M, Faillon JM, Dujon A, Lajos PS, Riquet M, Jancovici R. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000 May;69(5):1525-8.
- Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996 Jun;61(6):1636-40.
- Suen HC, Mathisen DJ, Grillo HC, LeBlanc J, McLoud TC, Moncure AC, Hilgenberg AD. Surgical management and radiological characteristics of bronchogenic cysts. Ann Thorac Surg 1993 55(2):476-81.
- Cioffi U, Bonavina L, De Simone M, Santambrogio L, Pavoni G, Testori A, Peracchia A. Presentation and surgical management of bronchogenic and esophageal duplication cysts in adults. Chest. 1998 Jun;113(6):1492-6.
- Gustafson RA, Murray GF, Warden HE, Hill RC, Rozar GE. Intralobar sequestration. A missed diagnosis. Ann Thorac Surg 1989 47(6):841-7.
- Bratu I, Flageole H, Chen MF, Di Lorenzo M, Yazbeck S, Laberge JM. The multiple facets of pulmonary sequestration. J Pediatr Surg. 2001 May;36(5):784-90.
- Bailey PV, Tracy T Jr, Connors RH, deMello D, Lewis JE, Weber TR. Congenital bronchopulmonary malformations. Diagnostic and therapeutic considerations. J Thorac Cardiovasc Surg 1990 99(4):597-602.
- Winters WD, Effmann EL. Congenital masses of the lung: prenatal and postnatal imaging evaluation. J Thorac Imaging. 2001 Oct;16(4):196-206.