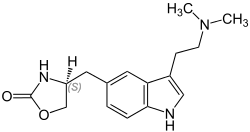Zolmitriptan (nasal spray)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Turky Alkathery, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Zolmitriptan (nasal spray) is a serotonin (5‑HT) 1B/1D receptor agonist that is FDA approved for the treatment of migraine with or without aura in adults. Common adverse reactions include unusual taste, paresthesia, dizziness, and hyperesthesia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
- ZOMIG Nasal Spray is indicated for the acute treatment of migraine with or without aura in adults.
- Limitations of Use:
- Only use zolmitriptan if a clear diagnosis of migraine has been established. If a patient has no response to ZOMIG treatment for the first migraine attack, reconsider the diagnosis of migraine before zolmitriptan is administered to treat any subsequent attacks.
- ZOMIG is not indicated for the prevention of migraine attacks.
- Safety and effectiveness of ZOMIG have not been established for cluster headache.
- Not recommended in patients with moderate or severe hepatic impairment.
Dosage
Dosing Information
- The recommended starting dose for ZOMIG nasal spray is 2.5 mg. As the individual response to zolmitriptan Nasal spray may vary, the dose should be adjusted on an individual basis. The maximum recommended single dose of ZOMIG is 5 mg.
- In controlled clinical trials, a greater proportion of patients had headache response following a 2.5 mg or 5 mg dose than following a 1 mg dose. There was little added benefit from the 5 mg dose compared to the 2.5 mg dose, but adverse reactions were more frequent with the 5 mg dose.
- If the migraine has not resolved by 2 hours after taking ZOMIG, or returns after a transient improvement, another dose may be administered at least 2 hours after the previous dose.
- The maximum daily dose should not exceed 10 mg in any 24‑hour period.
- The safety of zolmitriptan in the treatment of an average of more than four headaches in a 30‑day period has not been established.
Dosing in Patients with Hepatic Impairment
- Zolmitriptan nasal spray is not recommended in patients with moderate to severe hepatic impairment because of increased zolmitriptan blood levels in these patients and elevation of blood pressure in some of these patients. The recommended dosage of zolmitriptan nasal spray in patients with mild hepatic impairment is the same as for patients with normal hepatic function.
Dosing in Patients taking Cimetidine
- If zolmitriptan is co‑administered with cimetidine, limit the maximum single dose of zolmitriptan to 2.5 mg, not to exceed 5 mg in any 24‑hour period.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Zolmitriptan (nasal spray) in adult patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Zolmitriptan (nasal spray) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness in pediatric patients have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
- There is limited information regarding Off-Label Guideline-Supported Use of Zolmitriptan (nasal spray) in pediatric patients.
Non–Guideline-Supported Use
- There is limited information regarding Off-Label Non–Guideline-Supported Use of Zolmitriptan (nasal spray) in pediatric patients.
Contraindications
- Ischemic coronary artery disease (angina pectoris, history of myocardial infarction, or documented silent ischemia), other significant underlying cardiovascular disease, or coronary artery vasospasm including Prinzmetal’s Angina.
- Wolff-Parkinson White Syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders
- History of stroke, transient ischemic attack (TIA) or history of hemiplegic or basilar migraine because these patients are at higher risk of stroke
- Peripheral vascular disease (PVD)
- Ischemic bowel disease
- Uncontrolled hypertension
- Recent use (i.e., within 24 hours) of another 5-HT1 agonist, ergotamine-containing medication, or ergot type medication (such as dihydroergotamine or methysergide)
- Concurrent administration of an MAO A inhibitor or recent discontinuation of a MAO A inhibitor (that is within 2 weeks)
- Known hypersensitivity to zolmitriptan (angioedema and anaphylaxis seen)
Warnings
Myocardial Ischemia, Myocardial Infarction, and Prinzmetal’s Angina
- Zolmitriptan is contraindicated in patients with ischemic or vasospastic coronary artery disease (CAD). There have been rare reports of serious cardiac adverse reactions, including acute myocardial infarction, occurring within a few hours following administration of zolmitriptan. Some of these reactions occurred in patients without known CAD. 5-HT1 agonists including zolmitriptan may cause coronary artery vasospasm (Prinzmetal’s Angina), even in patients without a history of CAD.
- Perform a cardiovascular evaluation in triptan naïve patients who have multiple cardiovascular risk factors (e.g., increased age, diabetes, hypertension, smoking, obesity, strong family history of CAD) prior to receiving zolmitriptan. Do not administer zolmitriptan if there is evidence of CAD or coronary artery vasospasm. For patients with multiple cardiovascular risk factors who have a negative cardiovascular evaluation, consider administrating the first zolmitriptan dose in a medically-supervised setting and performing an electrocardiogram (ECG) immediately following zolmitriptan administration. For such patients, consider periodic cardiovascular evaluation in intermittent long-term users of zolmitriptan.
Arrhythmias
- Life‑threatening disturbances of cardiac rhythm including ventricular tachycardia and ventricular fibrillation leading to death have been reported within a few hours following the administration of 5‑HT1 agonists. Discontinue zolmitriptan if these disturbances occur. Patients with Wolff-Parkinson‑White Syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders should not receive zolmitriptan.
Chest, Throat, Neck and/or Jaw Pain/Tightness/Pressure
- As with other 5‑HT1 agonists, sensations of tightness, pain, pressure, and heaviness in the precordium, throat, neck, and jaw commonly occur after treatment with zolmitriptan and is usually non-cardiac in origin. However, if a cardiac origin is suspected, patients should be evaluated. Patients shown to have CAD and those with Prinzmetal’s variant angina should not receive 5 HT1 agonists.
Cerebrovascular Events
- Cerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5‑HT1 agonists, and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the 5‑HT1 agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine, when they were not. Discontinue zolmitriptan if a cerebrovascular event occurs.
- As with other acute migraine therapies, before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with symptoms atypical for migraine, other potentially serious neurological conditions should be excluded. Zolmitriptan should not be administered to patients with a history of stroke or transient ischemic attack.
Other Vasospasm Reactions
- 5‑HT1 agonists, including zolmitriptan, may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), splenic infarction, and Raynaud’s syndrome. In patients who experience symptoms or signs suggestive of vasospasm reaction following the use of any 5‑HT1 agonist, the suspected vasospasm reaction should be ruled out before receiving additional zolmitriptan doses.
- Reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5‑HT1 agonists. Since visual disorders may be part of a migraine attack, a causal relationship between these events and the use of 5‑HT1 agonists have not been clearly established.
Medication Overuse Headache
- Overuse of acute migraine drugs (e.g. ergotamine, triptans, opioids, or a combination of drugs for 10 or more days per month) may lead to exacerbation of headache (medication overuse headache). Medication overuse headache may present as migraine-like daily headaches, or as a marked increase in frequency of migraine attacks. Detoxification of patients, including withdrawal of the overused drugs, and treatment of withdrawal symptoms (which often includes a transient worsening of headache) may be necessary.
Serotonin Syndrome
- Serotonin syndrome may occur with triptans, including zolmitriptan, particularly during co-administration with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and MAO inhibitors. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms usually rapidly occurs within minutes to hours of receiving a new or a greater dose of a serotonergic medication. zolmitriptan treatment should be discontinued if serotonin syndrome is suspected.
Increase in Blood Pressure
- Significant elevations in systemic blood pressure have been reported in patients treated with 5‑HT1 agonists including patients without a history of hypertension. Very rarely these increases in blood pressure have been associated with significant clinical events. In healthy subjects treated with 5 mg of zolmitriptan oral tablet, an increase of 1 and 5 mm Hg in the systolic and diastolic blood pressure, respectively, was seen. In a study of patients with moderate to severe liver impairment, 7 of 27 patients experienced 20 to 80 mm Hg elevations in systolic and/or diastolic blood pressure after a dose of 10 mg of zolmitriptan oral tablet. As with all triptans, blood pressure should be monitored in zolmitriptan‑treated patients. Zolmitriptan is contraindicated in patients with uncontrolled hypertension.
Adverse Reactions
Clinical Trials Experience
- Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
- Among 460 patients treating 1180 single attacks with zolmitriptan nasal spray in a blinded placebo controlled trial (Study 1), there was a low withdrawal rate related to adverse reactions: 5 mg (1.3%), 2.5 mg (0%) and placebo (0.4%). None of the withdrawals were due to a serious event. One patient was withdrawn due to abnormal ECG changes from baseline that were incidentally found 23 days after the last dose of zolmitriptan Nasal Spray.
- The most common adverse reactions (≥5% and > placebo) in any dosage strength in clinical trials for zolmitriptan Nasal Spray were: unusual taste, paresthesia, hyperesthesia, and dizziness. The incidence of adverse reactions was generally dose‑related.
- Table 1 lists the adverse reactions from the controlled clinical trial (Study 1) that occurred in ≥2% of patients in either the 2.5 or 5 mg zolmitriptan nasal spray dose groups and with an incidence greater than placebo.
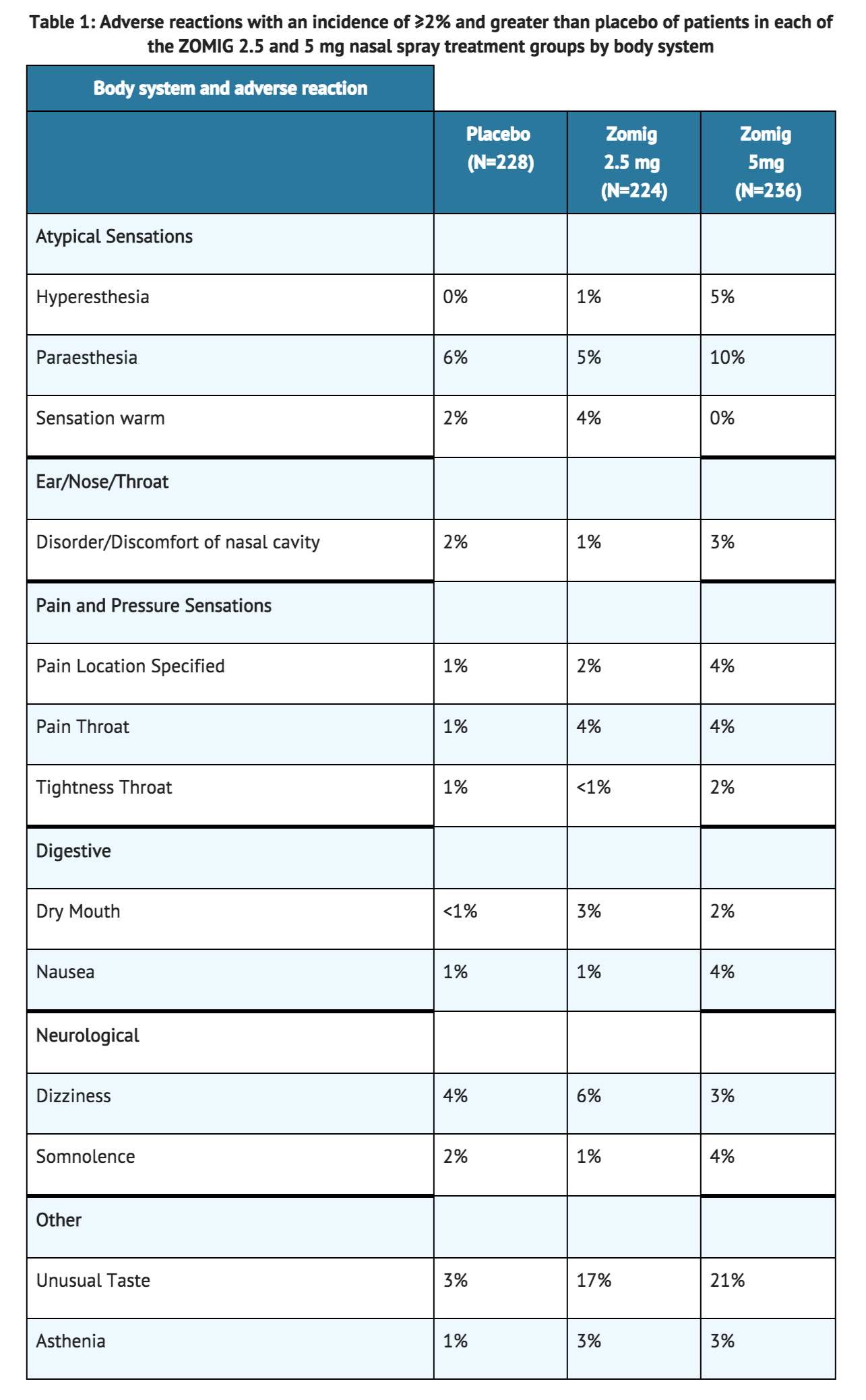
- In Study 1, adverse clinical reactions occurring in ≥1% and < 2% of patients in all attacks in either zolmitriptan nasal spray dose group and with incidence greater than that of placebo were abdominal pain, chills, pressure throat, edema face, pressure chest, palpitation, dysphagia, arthralgia, myalgia, and depersonalization.
- The incidence of adverse reactions in controlled clinical trials was not affected by gender, weight, or age of the patients (18 39 vs. 40 65 years of age), or presence of aura. There were insufficient data to assess the impact of race on the incidence of adverse reactions.
Local Adverse Reactions:
- Among 460 patients using zolmitriptan 2.5 mg or 5 mg in the controlled clinical trial, approximately 3% noted local irritation or soreness at the site of administration. Adverse reactions of any kind, perceived in the nasopharynx (which may include systemic effects of triptans) were severe in about 1% of patients and approximately 57% resolved in 1 hour. Nasopharyngeal examinations, in a subset of patients participating in two long term trials of up to one year duration, failed to demonstrate any clinically significant changes with repeated use of zolmitriptan Nasal Spray.
- All nasopharyngeal adverse reactions with an incidence of ≥ 2% of patients in any zolmitriptan nasal spray dose groups are included in ADVERSE REACTIONS Table 1.
Other Adverse Reactions:
- In the paragraphs that follow, the frequencies of less commonly reported adverse clinical reactions are presented. Because the reports include reactions observed in open and uncontrolled studies, the role of zolmitriptan in their causation cannot be reliably determined. Furthermore, variability associated with adverse reaction reporting, the terminology used to describe adverse reactions, etc., limit the value of the quantitative frequency estimates provided. Reaction frequencies are calculated as the number of patients who used zolmitriptan Nasal Spray and reported a reaction divided by the total number of patients exposed to zolmitriptan Nasal Spray (n=3059). All reported reactions are included except those already listed in the previous table, those too general to be informative, and those not reasonably associated with the use of the drug. Reactions are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: infrequent adverse reactions are those occurring in 1/100 to 1/1,000 patients and rare adverse reactions are those occurring in fewer than 1/1,000 patients.
- General: Infrequent: allergic reactions.
- Cardiovascular: Infrequent: arrhythmias, hypertension, syncope and tachycardia. Rare: angina pectoris and myocardial infarct.
- Digestive: Rare: stomatitis.
- Neurological: Infrequent: agitation, amnesia, anxiety, depression, insomnia, and nervousness. Rare: convulsions.
- Respiratory: Infrequent: bronchitis, increased cough, dyspnea, epistaxis, laryngeal edema, pharyngitis, rhinitis, and sinusitis.
- Urogenital: Infrequent: polyuria and urinary urgency. Rare: urinary frequency.
- Special senses: Infrequent: tinnitus. Rare: conjunctivitis, dry eye, and visual field defect.
- The adverse experience profile seen with zolmitriptan Nasal Spray is similar to that seen with zolmitriptan tablets and zolmitriptan/ZMT tablets except for the occurrence of local adverse reactions from the nasal spray (see zolmitriptan tablet/zolmitriptan‑ZMT oral disintegrating tablet Prescribing Information).
Postmarketing Experience
- The following adverse reactions were identified during post approval use of zolmitriptan. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- The reactions enumerated include all except those already listed in the Clinical Trials Experience section above or the Warnings and Precautions section.
Hypersensitivity Reactions:
- As with other 5 HT1B/1D agonists, there have been reports of anaphylaxis, anaphylactoid, and hypersensitivity reactions including angioedema in patients receiving zolmitriptan. Zolmitriptan is contraindicated in patients with a history of hypersensitivity reaction to zolmitriptan.
Drug Interactions
Ergot-containing drugs
- Ergot-containing drugs have been reported to cause prolonged vasospastic reactions. Because these effects may be additive, use of ergotamine‑containing or ergot‑type medications (like dihydroergotamine or methysergide) and zolmitriptan within 24 hours of each other is contraindicated.
MAO-A Inhibitors
- MAO-A inhibitors increase the systemic exposure of zolmitriptan. Therefore, the use of zolmitriptan in patients receiving MAO-A inhibitors is contraindicated.
5-HT1B/1D agonists (e.g. triptans)
- Concomitant use of other 5-HT1B/1D agonists (including triptans) within 24 hours of zolmitriptan treatment is contraindicated because the risk of vasospastic reactions may be additive.
Cimetidine
- Following administration of cimetidine, the half‑life and AUC of zolmitriptan and its active metabolites were approximately doubled. If cimetidine and zolmitriptan are used concomitantly, limit the maximum single dose of zolmitriptan to 2.5 mg, not to exceed 5 mg in any 24 hour period.
Selective Serotonin Reuptake Inhibitors/Serotonin Norepinephrine Reuptake Inhibitors and Serotonin Syndrome
- Cases of life-threatening serotonin syndrome have been reported during combined use of selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs) and triptans.
Use in Specific Populations
Pregnancy
- There are no adequate and well controlled studies in pregnant women; therefore, zolmitriptan should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. In reproductive toxicity studies in rats and rabbits, oral administration of zolmitriptan to pregnant animals resulted in embryolethality and fetal abnormalities (malformations and variations) at clinically relevant exposures.
- When zolmitriptan was administered to pregnant rats during the period of organogenesis at oral doses of 100, 400, and 1200 mg/kg/day (plasma exposures (AUCs) ≈280, 1100, and 5000 times the human AUC at the maximum recommended human dose (MRHD) of 10 mg/day), there was a dose-related increase in embryolethality. A no-effect dose for embryolethality was not established. When zolmitriptan was administered to pregnant rabbits during the period of organogenesis at oral doses of 3, 10, and 30 mg/kg/day (plasma AUCs ≈1, 11, and 42 times the human AUC at the MRHD), there were increases in embryolethality and in fetal malformations and variations. The no-effect dose for adverse effects on embryo-fetal development was associated with a plasma AUC similar to that in humans at the MRHD. When female rats were given zolmitriptan during gestation, parturition, and lactation at oral doses of 25, 100, and 400 mg/kg/day (plasma AUCs ≈70, 280, and 1100 times that in human at the MRHD), an increased incidence of hydronephrosis was found in the offspring. The no-effect dose was associated with a plasma AUC ≈280 times that in humans at the MRHD.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Zolmitriptan (nasal spray) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Zolmitriptan (nasal spray) during labor and delivery.
Nursing Mothers
- It is not known whether zolmitriptan is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from zolmitriptan, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. In rats, oral dosing with zolmitriptan resulted in levels in milk up to 4 times higher than in plasma.
Pediatric Use
- Safety and effectiveness in pediatric patients have not been established.
- A single, multicenter, double-blind, randomized placebo-controlled, study was conducted to evaluate the efficacy of zolmitriptan 5 mg nasal spray in the acute treatment of migraine headache in 171 evaluable adolescent subjects 12 to 17 years of age. Efficacy was not established in that study.
- Adverse reactions observed in this study were similar in nature and frequency to those reported in clinical trials of zolmitriptan Nasal Spray in adults. The most commonly reported adverse reactions (≥ 2% and > placebo) were dysgeusia (7%), nasal discomfort (3%), dizziness (2%), nasal congestion (2%), nausea (2%), and throat irritation (2%).
- Zolmitriptan Nasal Spray has not been studied in pediatric patients age 11 years and under.
- In the postmarketing experience with triptans, including zolmitriptan, there is a limited number of reports that describe pediatric patients who have experienced clinically serious adverse events; those that were reported are similar in nature to those reported rarely in adults.
Geriatic Use
- Clinical studies of zolmitriptan did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Geriatric patients who have other cardiovascular risk factors (e.g., diabetes, hypertension, smoking, obesity, strong family history of coronary artery disease) should have a cardiovascular evaluation prior to receiving zolmitriptan. The pharmacokinetics of zolmitriptan were similar in geriatric patients (aged > 65 years) compared to younger.
Gender
There is no FDA guidance on the use of Zolmitriptan (nasal spray) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Zolmitriptan (nasal spray) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Zolmitriptan (nasal spray) in patients with renal impairment.
Hepatic Impairment
- The effect of hepatic disease on the pharmacokinetics of zolmitriptan nasal spray has not been evaluated. After oral administration, zolmitriptan blood levels were increased in patients with moderate to severe hepatic impairment, and significant elevation in blood pressure was observed in some of these patients. Zolmitriptan nasal spray is not recommended in patients with moderate to severe hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Zolmitriptan (nasal spray) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Zolmitriptan (nasal spray) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Nasal.
Monitoring
- The elimination half-life of zolmitriptan is 3 hours and therefore monitoring of patients after overdose with zolmitriptan should continue for at least 15 hours or while symptoms or signs persist.
- Monitoring and support of the cardiovascular system in overdose.
IV Compatibility
- There is limited information regarding IV Compatibility.
Overdosage
- There is no experience with acute overdose. Clinical study subjects receiving single 50 mg oral doses of zolmitriptan commonly experienced sedation.
- The elimination half-life of zolmitriptan is 3 hours and therefore monitoring of patients after overdose with zolmitriptan should continue for at least 15 hours or while symptoms or signs persist.
- There is no specific antidote to zolmitriptan. In cases of severe intoxication, intensive care procedures are recommended, including establishing and maintaining a patent airway, ensuring adequate oxygenation and ventilation, and monitoring and support of the cardiovascular system.
- It is unknown what effect hemodialysis or peritoneal dialysis has on the plasma concentrations of zolmitriptan.
Pharmacology
Mechanism of Action
- Zolmitriptan binds with high affinity to human recombinant 5‑HT1D and 5‑HT1B receptors, and moderate affinity for 5‑HT1A receptors. The N‑desmethyl metabolite also has high affinity for 5‑HT1B/1D and moderate affinity for 5‑HT1A receptors.
- Current theories proposed to explain the etiology of migraine headache suggest that symptoms are due to local cranial vasodilatation and/or to the release of sensory neuropeptides (vasoactive intestinal peptide, substance P and calcitonin gene-related peptide) through nerve endings in the trigeminal system. The therapeutic activity of zolmitriptan for the treatment of migraine headache is thought to be due to the agonist effects at the 5‑HT1B/1D receptors on intracranial blood vessels (including the arterio‑venous anastomoses) and sensory nerves of the trigeminal system which result in cranial vessel constriction and inhibition of pro-inflammatory neuropeptide release.
Structure
- zolmitriptan nasal spray contains zolmitriptan, which is a selective 5-hydroxytryptamine 1B/1D (5-HT1B/1D) receptor agonist. Zolmitriptan is chemically designated as (S)-4-[3-[2-(dimethylamino)ethyl]-1H-indol-5-yl]methyl]-2-oxazolidinone and has the following chemical structure:
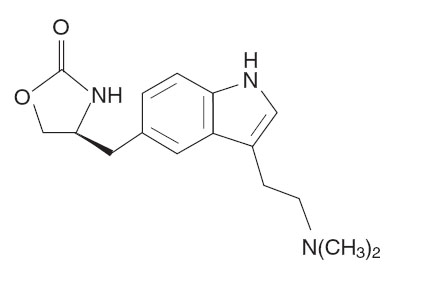
- The empirical formula is C16H21N3O2, representing a molecular weight of 287.36. Zolmitriptan is a white to almost white powder that is readily soluble in water. Zolmitriptan Nasal Spray is supplied as a clear to pale yellow solution of zolmitriptan, buffered to a pH 5.0. Each zolmitriptan Nasal Spray contains 2.5 mg or 5 mg of zolmitriptan in a 100‑µL unit‑dose aqueous buffered solution containing citric acid, anhydrous, USP, disodium phosphate dodecahydrate USP and purified water USP.
- Zolmitriptan Nasal Spray is hypertonic. The osmolarity of zolmitriptan Nasal Spray for 2.5 mg is 360 to 420 mOsmol, and for 5 mg is 420 to 470 mOsmol.
Pharmacodynamics
Absorption:
- Zolmitriptan nasal spray is rapidly absorbed via the nasopharynx as detected in a Photon Emission Tomography (PET) study using 11C zolmitriptan. The mean relative bioavailability of the nasal spray formulation is 102%, compared with the oral tablet. Zolmitriptan was detected in plasma by 5 minutes and peak plasma concentration generally was achieved by 3 hours. The time at which maximum plasma concentrations were observed was similar after single (1 day) or multiple (4 days) nasal dosing. Plasma concentrations of zolmitriptan are sustained for 4 to 6 hours after dosing. Zolmitriptan and its active N-desmethyl metabolite display linear kinetics after single or multiple doses of zolmitriptan nasal spray over the dose range of 0.1 to 10 mg.
- The pharmacokinetics of the N desmethyl metabolite are similar to that of zolmitriptan for all nasal spray dosages. The N desmethyl metabolite is detected in plasma by 15 minutes and peak plasma concentration is generally achieved by 3 hours after administration. Food has no significant effect on the bioavailability of zolmitriptan.
Distribution:
- Plasma protein binding of zolmitriptan is 25% over the concentration range of 10‑1000 ng/mL. The mean apparent volume of distribution for zolmitriptan nasal spray formulation is 8.4 L/kg.
Metabolism:
- Zolmitriptan is converted to an active N‑desmethyl metabolite such that the metabolite concentrations are about two-thirds that of zolmitriptan. Because the 5HT1B/1D potency of the metabolite is 2 to 6 times that of the parent compound, the metabolite may contribute a substantial portion of the overall effect after zolmitriptan administration.
Excretion:
- The mean elimination half life for zolmitriptan and N‑desmethyl metabolite following single or multiple nasal spray administration are approximately 3 hours, similar to the half‑life values seen after oral tablet administration.
- In a study with orally administered zolmitriptan, total radioactivity recovered in urine and feces was 65% and 30% of the administered dose, respectively. In urine, unchanged zolmitriptan and N‑desmethyl metabolite accounted for 8% and 4% of the dose, respectively, whereas the inactive indole acetic acid and N‑oxide metabolites accounted for 31% and 7% of the dose, respectively.
- Mean total plasma clearance for zolmitriptan nasal spray is 25.9 mL/min/kg, of which one-sixth is renal clearance. The renal clearance is greater than the glomerular filtration rate suggesting renal tubular secretion.
Special Populations:
- Age:
- The pharmacokinetics of orally administered zolmitriptan in healthy elderly non‑migraineur volunteers (age 65‑76 yrs) was similar to those in younger non‑migraineur volunteers (age 18‑39 yrs).
- Sex:
- Mean plasma concentrations of orally administered zolmitriptan were up to 1.5‑fold higher in females than males.
- Race:
- There are no significant difference in the pharmacokinetics of orally administered zolmitriptan in Japanese and Caucasians.
- Renal Impairment:
- The effect of renal impairment on the pharmacokinetics of zolmitriptan nasal spray has not been evaluated. After orally dosing zolmitriptan, renal clearance was reduced by 25% in patients with severe renal impairment (Clcr ≥ 5 ≤ 25 mL/min) compared with the normal group (Clcr ≥ 70 mL/min); no significant change in clearance was observed in the moderately renally impaired group (Clcr ≥ 26 ≤ 50 mL/min).
- Hepatic Impairment:
- The effect of hepatic disease on the pharmacokinetics of zolmitriptan nasal spray has not been evaluated. In patients with severe hepatic impairment, the mean Cmax, Tmax, and AUC of zolmitriptan dosed orally were increased 1.5‑fold, 2‑fold (2 vs. 4 hours), and 3‑fold, respectively, compared to subjects with normal hepatic function. Seven out of 27 patients experienced 20 to 80 mm Hg elevations in systolic and/or diastolic blood pressure after a 10 mg zolmitriptan dose.
- Hypertensive Patients:
- No differences in the pharmacokinetics of oral zolmitriptan or its effects on blood pressure were seen in mild‑to‑moderate hypertensive volunteers compared with normotensive controls.
- Drug Interactions:
- All drug interaction studies were performed in healthy volunteers using a single 10 mg dose of zolmitriptan and a single dose of the other drug except where otherwise noted. Eight drug interaction studies have been performed with zolmitriptan tablets and one study (xylometazoline) was performed with nasal spray.
- Xylometazoline:
- An in vivo drug interaction study with zolmitriptan Nasal Spray indicated that 1 spray (100µL dose) of xylometazoline (0.1% w/v), a decongestant, administered 30 minutes prior to a 5 mg nasal dose of zolmitriptan did not alter the pharmacokinetics of zolmitriptan.
- Fluoxetine:
- The pharmacokinetics of zolmitriptan, as well as its effect on blood pressure, were unaffected by 4 weeks of pre‑treatment with oral fluoxetine (20 mg/day).
- MAO Inhibitors:
- Following one week of administration of moclobemide (150 mg twice‑daily), a specific MAO‑A inhibitor, there was an increase of about 25% in both Cmax and AUC for zolmitriptan and a 3‑fold increase in the Cmax and AUC of the active N‑desmethyl metabolite of zolmitriptan.
- Selegiline, a selective MAO‑B inhibitor, at a dose of 10 mg/day for 1 week, had no effect on the pharmacokinetics of zolmitriptan and its metabolite.
- Propranolol:
- Cmax and AUC of zolmitriptan increased 1.5‑fold after one week of dosing with propranolol (160 mg/day). Cmax and AUC of the N‑desmethyl metabolite were reduced by 30% and 15%, respectively. There were no interactive effects on blood pressure or pulse rate following administration of propranolol with zolmitriptan.
- Acetaminophen:
- A single 1g dose of acetaminophen does not alter the pharmacokinetics of zolmitriptan and its N‑desmethyl metabolite. However, zolmitriptan delayed the Tmax of acetaminophen by one hour.
- Metoclopramide:
- A single 10 mg dose of metoclopramide had no effect on the pharmacokinetics of zolmitriptan or its metabolites.
- Oral Contraceptives:
- Retrospective analysis of pharmacokinetic data across studies indicated that mean Cmax and AUC of zolmitriptan were 30% and 50% higher, respectively, and Tmax was delayed by one-half hour in females taking oral contraceptives compared to females not taking oral contraceptives. The effect of zolmitriptan on the pharmacokinetics of oral contraceptives has not been studied.
- Cimetidine:
- Following the administration of cimetidine, the half-life and AUC of a 5 mg dose of zolmitriptan and its active metabolite were approximately doubled. A dosage adjustment is therefore required.
Pharmacokinetics
- There is limited information regarding pharmacokinetics.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis:
- Zolmitriptan was administered to mice and rats at doses up to 400 mg/kg/day. Mice were dosed for 85 weeks (males) and 92 weeks (females); rats were dosed for 101 weeks (males) and 86 weeks (females). There was no evidence of drug-induced tumors in mice at plasma exposures (AUC) up to approximately 700 times that in humans at the maximum recommended human dose (MRHD) of 10 mg/day. In rats, there was an increase in the incidence of thyroid follicular cell hyperplasia and thyroid follicular cell adenomas in male rats receiving 400 mg/kg/day. No increase in tumors was observed in rats at 100 mg/kg/day, a dose associated with a plasma AUC ≈700 times that in humans at the MRHD.
Mutagenesis:
- Zolmitriptan was positive in an in vitro bacterial reverse mutation (Ames) assay and in an in vitro chromosomal aberration assay in human lymphocytes. Zolmitriptan was negative in an in vitro mammalian gene cell mutation (CHO/HGPRT) assay and in oral in vivo micronucleus assays in mouse and rat.
Impairment of Fertility:
- Studies of male and female rats administered zolmitriptan prior to and during mating and up to implantation showed no impairment of fertility at oral doses up to 400 mg/kg/day. The plasma exposure (AUC) at this dose was approximately 3000 times that in humans at the MRHD.
Clinical Studies
- The efficacy of zolmitriptan Nasal Spray 2.5 and 5 mg in the acute treatment of migraine headache with or without aura was demonstrated in Study 1, a randomized, outpatient, double blind, placebo-controlled trial.
- In Study 1, patients were instructed to treat a moderate to severe headache. headache response, defined as a reduction in headache severity from moderate or severe pain to mild or no pain, was assessed 15, 30, 45 minutes and 1, 2, and 4 hours after dosing. Pain free response rates and associated symptoms such as nausea, photophobia, and phonophobia were also assessed. A dose of escape medication was allowed 4 to 24 hours after the initial treatment for persistent and recurrent headache.
- In Study 1, of the patients taking zolmitriptan 2.5 or 5 mg, 83% were female and 99% were Caucasian, with a mean age of 41 years (range 18 to 65 years).
- The two-hour headache response rates in patients treated with zolmitriptan Nasal Spray were significantly higher among patients receiving zolmitriptan Nasal Spray at all doses, compared with placebo (see Table 2).
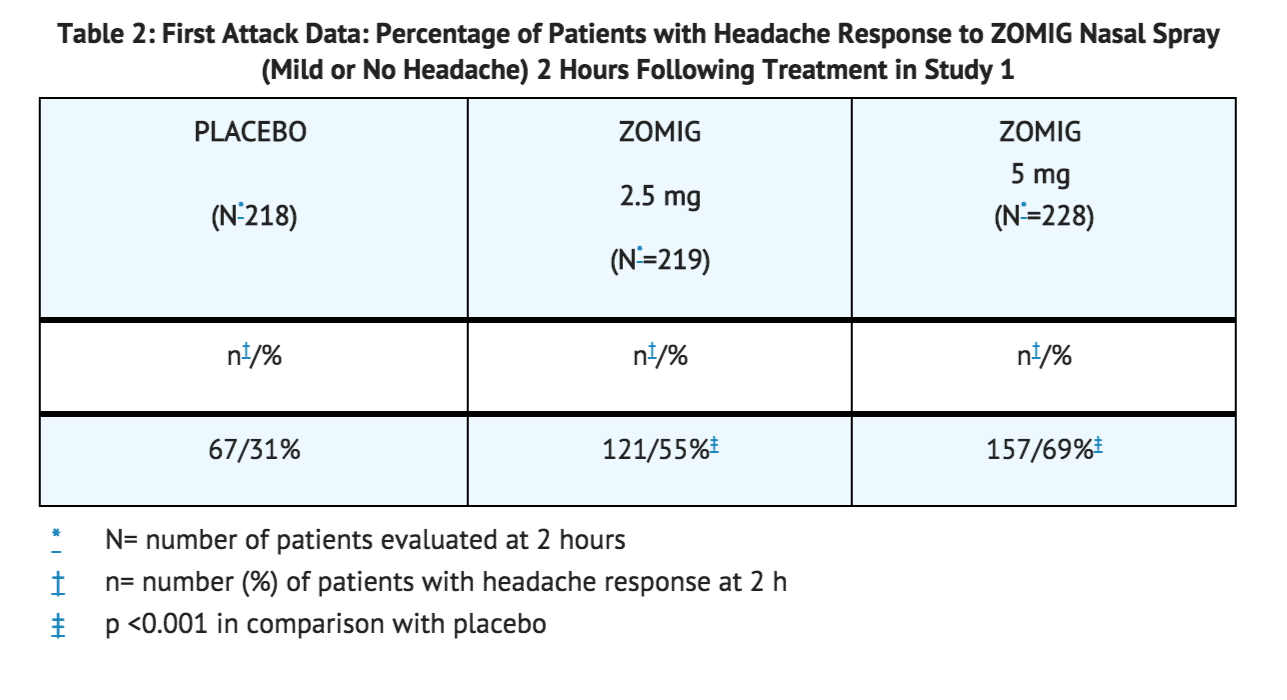
- The estimated probability of achieving an initial headache response following treatment with zolmitriptan Nasal Spray is depicted in Figure 1.
Figure 1: Estimated probability of achieving an initial headache response after treatment in Study 1
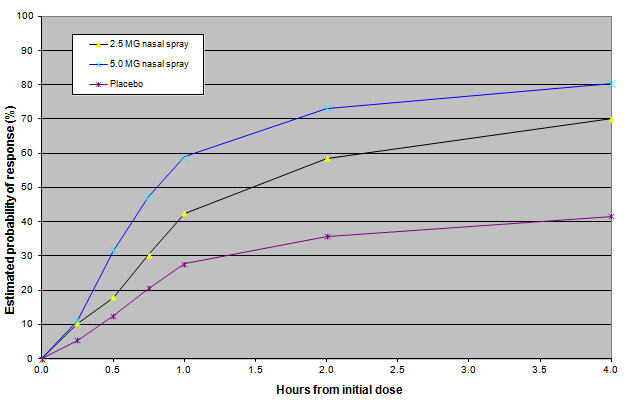
- Note: Figure 1 shows the Kaplan-Meier plot of the probability over time of obtaining headache response (moderate or severe headache improving to mild or no pain) following treatment with zolmitriptan nasal spray. The estimates displayed are based on a placebo-controlled, outpatient trial providing evidence of efficacy. Patients not achieving headache response or taking additional treatment prior to 4 hours were censored to 4 hours.
- For patients with migraine associated photophobia, phonophobia, and nausea at baseline, there was a decreased incidence of these symptoms following administration of zolmitriptan Nasal Spray as compared with placebo.
- Four to 24 hours following the initial dose of study treatment, patients were allowed to use additional treatment for pain relief in the form of a second dose of study treatment or other medication. The estimated probability of patients taking a second dose or other medication for migraine over the 24 hours following the initial dose of study treatment is summarized in Figure 2.
- Figure 2: Estimated probability of patients taking an escape medication within the 24 hours following the initial dose of study treatment in Study 1
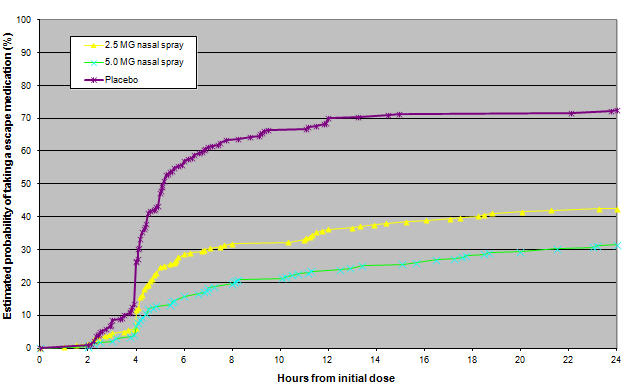
- This Kaplan-Meier plot is based on data obtained from the placebo controlled clinical trial. Patients not using additional treatments were censored at 24 hours. The plot includes both patients who had headache response at 2 hours and those who had no response to the initial dose. It should be noted that the protocol did not allow remedication within 4 hours post dose.
- The efficacy of zolmitriptan was unaffected by presence of aura; presence of headache upon awakening, relationship to menses; gender, age or weight of the patient; or presence of pre‑treatment nausea.
- The efficacy of zolmitriptan Nasal Spray 5 mg was further supported by an interim analysis of another similarly designed trial. The 2 hour headache response rates for the first 210 subjects in that study for zolmitriptan 5 mg and placebo were 70% and 47%, respectively (N=108 and 102, respectively, p=0.0006).
How Supplied
- The zolmitriptan Nasal Spray device is a blue colored plastic device with a gray protection cap, labeled to indicate the nominal dose. Each zolmitriptan Nasal Spray device administers a single dose of zolmitriptan.
- ZOMIG Nasal Spray is supplied as a clear to pale yellow solution of zolmitriptan, buffered to a pH 5.0. Each ZOMIG Nasal Spray device contains 2.5 mg or 5 mg of zolmitriptan in a 100 µL unit dose aqueous buffered solution containing citric acid, anhydrous, USP, disodium phosphate dodecahydrate USP and purified water USP.
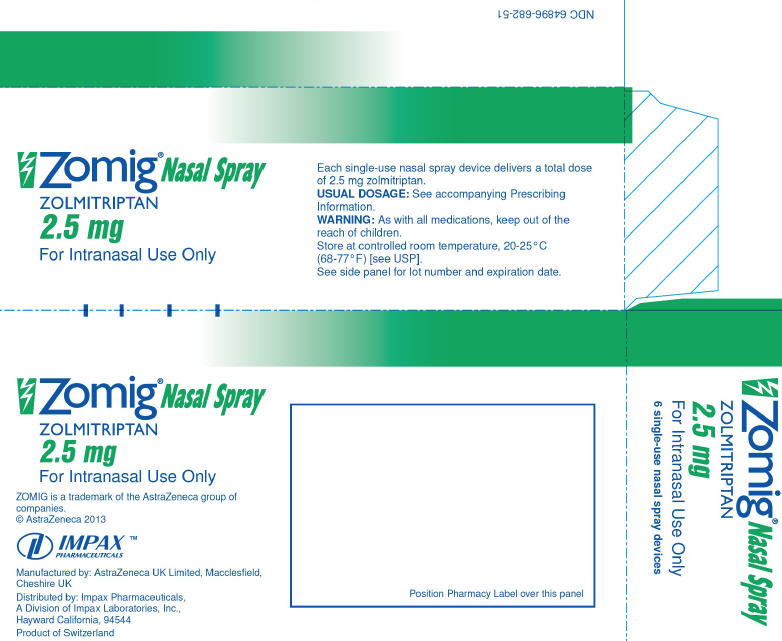
- 2.5 mg ZOMIG® Nasal Spray is supplied in boxes of 6 single‑use nasal spray units. (NDC 64896-682-51)
- 5 mg ZOMIG® Nasal Spray is supplied in boxes of 6 single‑use nasal spray units. (NDC 64896-681-51).
- Each ZOMIG® Nasal Spray single dose unit spray supplies 2.5 and 5 mg, respectively, of zolmitriptan. The ZOMIG® Nasal Spray unit must be discarded after use.
Storage
- Store at controlled room temperature, 20‑25°C (68‑77°F) [see USP].
Images
Drug Images
{{#ask: Page Name::Zolmitriptan (nasal spray) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Zolmitriptan (nasal spray) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
See FDA-Approved Patient Labeling (Patient Information)
- Inform patients that ZOMIG may cause serious cardiovascular side effects such as myocardial infarction or stroke, which may result in hospitalization and even death. Although serious cardiovascular events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and should ask for medical advice when observing any indicative sign or symptoms.
Medication Overuse Headache
- Inform patients that use of acute migraine drugs for 10 or more days per month may lead to an exacerbation of headache, and encourage patients to record headache frequency and drug use (e.g., by keeping a headache diary).
Serotonin Syndrome
- Inform patients about the risk of serotonin syndrome with the use of ZOMIG or other triptans, particularly during combined use with selective serotonin reuptake inhibitors (SSRIs) or serotonin norepinephrine reuptake inhibitors (SNRIs).
Pregnancy
- Inform patients that ZOMIG should not be used during pregnancy unless the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
- Advise patients to notify their healthcare provider if they are breastfeeding or plan to breastfeed.
Handling of ZOMIG nasal spray device
- The ZOMIG Nasal Spray device is packaged in a carton and is a blue colored plastic device with a gray protection cap, labeled to indicate the nominal dose. Caution patients to not remove the gray protection cap until prior to dosing. The ZOMIG Nasal Spray device is placed in a nostril and actuated to deliver a single dose. Caution patients to avoid spraying the contents of the device in their eyes.

Precautions with Alcohol
Alcohol-Zolmitriptan (nasal spray) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ZOMIG®[1]
Look-Alike Drug Names
- There is limited information regarding Look-Alike Drug Names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.