Zaleplon: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | {{DrugProjectFormSinglePage | ||
|authorTag={{DB}} | |authorTag={{DB}} | ||
|genericName=Zaleplon | |||
|aOrAn=a | |aOrAn=a | ||
|drugClass=anticonvulsant | |||
|indicationType=treatment | |||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=<!--Black Box Warning--> | |adverseReactions=<!--Black Box Warning--> | ||
| Line 110: | Line 113: | ||
<!--Contraindications--> | <!--Contraindications--> | ||
|contraindications=* | |contraindications=*Hypersensitivity to zaleplon or any excipients in the formulation (see also PRECAUTIONS). | ||
|warnings=* Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative/hypnotic drugs, including Sonata. Because some of the important adverse effects of Sonata appear to be dose-related, it is important to use the lowest possible effective dose, especially in the elderly (see DOSAGE AND ADMINISTRATION). | |||
A variety of abnormal thinking and behavior changes have been reported to occur in association with the use of sedative/hypnotics. Some of these changes may be characterized by decreased inhibition (e.g., aggressiveness and extroversion that seem out of character), similar to effects produced by alcohol and other CNS depressants. Other reported behavioral changes have included bizarre behavior, agitation, hallucinations, and depersonalization. | |||
Abnormal Thinking and Behavioral Changes | |||
Complex behaviors such as "sleep-driving" (i.e., driving while not fully awake after ingestion of a sedative-hypnotic, with amnesia for the event) have been reported. These events can occur in sedative-hypnotic-naive as well as in sedative-hypnotic-experienced persons. Although behaviors such as sleep-driving may occur with Sonata alone at therapeutic doses, the use of alcohol and other CNS depressants with Sonata appears to increase the risk of such behaviors, as does the use of Sonata at doses exceeding the maximum recommended dose. Due to the risk to the patient and the community, discontinuation of Sonata should be strongly considered for patients who report a "sleep-driving" episode. Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events. Amnesia and other neuropsychiatric symptoms may occur unpredictably. In primarily depressed patients, worsening of depression, including suicidal thoughts and actions (including completed suicides), has been reported in association with the use of sedative/hypnotics. | |||
It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above is drug induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation. | |||
Following rapid dose decrease or abrupt discontinuation of the use of sedative/hypnotics, there have been reports of signs and symptoms similar to those associated with withdrawal from other CNS-depressant drugs (see DRUG ABUSE AND DEPENDENCE). | |||
Sonata, like other hypnotics, has CNS-depressant effects. Because of the rapid onset of action, Sonata should only be ingested immediately prior to going to bed or after the patient has gone to bed and has experienced difficulty falling asleep. Patients receiving Sonata should be cautioned against engaging in hazardous occupations requiring complete mental alertness or motor coordination (e.g., operating machinery or driving a motor vehicle) after ingesting the drug, including potential impairment of the performance of such activities that may occur the day following ingestion of Sonata. Sonata, as well as other hypnotics, may produce additive CNS-depressant effects when coadministered with other psychotropic medications, anticonvulsants, antihistamines, narcotic analgesics, anesthetics, ethanol, and other drugs that themselves produce CNS depression. Sonata should not be taken with alcohol. Dosage adjustment may be necessary when Sonata is administered with other CNS-depressant agents because of the potentially additive effects. | |||
Severe anaphylactic and anaphylactoid reactions | |||
Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of sedative-hypnotics, including Sonata. Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis. Some patients have required medical therapy in the emergency department. If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal. Patients who develop angioedema after treatment with Sonata should not be rechallenged with the drug. | |||
====Precautions==== | ====Precautions==== | ||
* | *General | ||
Timing of Drug Administration | |||
Sonata should be taken immediately before bedtime or after the patient has gone to bed and has experienced difficulty falling asleep. As with all sedative/hypnotics, taking Sonata while still up and about may result in short-term memory impairment, hallucinations, impaired coordination, dizziness, and lightheadedness. | |||
Use in the elderly and/or debilitated patients | |||
Impaired motor and/or cognitive performance after repeated exposure or unusual sensitivity to sedative/hypnotic drugs is a concern in the treatment of elderly and/or debilitated patients. A dose of 5 mg is recommended for elderly patients to decrease the possibility of side effects (see DOSAGE AND ADMINISTRATION). Elderly and/or debilitated patients should be monitored closely. | |||
Use in patients with concomitant illness | |||
Clinical experience with Sonata in patients with concomitant systemic illness is limited. Sonata should be used with caution in patients with diseases or conditions that could affect metabolism or hemodynamic responses. | |||
Although preliminary studies did not reveal respiratory depressant effects at hypnotic doses of Sonata in normal subjects, caution should be observed if Sonata (zaleplon) is prescribed to patients with compromised respiratory function, because sedative/hypnotics have the capacity to depress respiratory drive. Controlled trials of acute administration of Sonata 10 mg in patients with mild to moderate chronic obstructive pulmonary disease or moderate obstructive sleep apnea showed no evidence of alterations in blood gases or apnea/hypopnea index, respectively. However, patients with compromised respiration due to preexisting illness should be monitored carefully. | |||
The dose of Sonata should be reduced to 5 mg in patients with mild to moderate hepatic impairment (see DOSAGE AND ADMINISTRATION). It is not recommended for use in patients with severe hepatic impairment. | |||
No dose adjustment is necessary in patients with mild to moderate renal impairment. Sonata has not been adequately studied in patients with severe renal impairment. | |||
Use in patients with depression | |||
As with other sedative/hypnotic drugs, Sonata should be administered with caution to patients exhibiting signs or symptoms of depression. Suicidal tendencies may be present in such patients and protective measures may be required. Intentional overdosage is more common in this group of patients (see OVERDOSAGE); therefore, the least amount of drug that is feasible should be prescribed for the patient at any one time. | |||
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity. | |||
Information for Patients | |||
A patient Medication Guide is also available for Sonata. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions that they may have. | |||
SPECIAL CONCERNS "Sleep-Driving" and other complex behaviors | |||
There have been reports of people getting out of bed after taking a sedative hypnotic medicine and driving their cars while not fully awake, often with no memory of the event. If a patient experiences such an episode, it should be reported to his or her doctor immediately, since "sleep-driving" can be dangerous. This behavior is more likely to occur when Sonata is taken with alcohol or other central nervous system depressants (see WARNINGS). Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sleep medicine. As with sleep-driving, patients usually do not remember these events. | |||
Laboratory Tests | |||
There are no specific laboratory tests recommended. | |||
<!--Adverse Reactions--> | |||
= | <!--Clinical Trials Experience--> | ||
|clinicalTrials=The premarketing development program for Sonata included zaleplon exposures in patients and/or normal subjects from 2 different groups of studies: approximately 900 normal subjects in clinical pharmacology/pharmacokinetic studies; and approximately 2,900 exposures from patients in placebo-controlled clinical effectiveness studies, corresponding to approximately 450 patient exposure years. The conditions and duration of treatment with Sonata varied greatly and included (in overlapping categories) open-label and double-blind phases of studies, inpatients and outpatients, and short-term or longer-term exposure. Adverse reactions were assessed by collecting adverse events, results of physical examinations, vital signs, weights, laboratory analyses, and ECGs. | |||
Adverse events during exposure were obtained primarily by general inquiry and recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse events without first grouping similar types of events into a smaller number of standardized event categories. In the tables and tabulations that follow, COSTART terminology has been used to classify reported adverse events. | |||
The stated frequencies of adverse events represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse event of the type listed. An event was considered treatment-emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation. | |||
Adverse Findings Observed in Short-Term, Placebo-Controlled Trials | |||
Adverse Events Associated With Discontinuation of Treatment | |||
In premarketing placebo-controlled, parallel-group phase 2 and phase 3 clinical trials, 3.1% of 744 patients who received placebo and 3.7% of 2,149 patients who received Sonata discontinued treatment because of an adverse clinical event. This difference was not statistically significant. No event that resulted in discontinuation occurred at a rate of ≥ 1%. | |||
Adverse Events Occurring at an Incidence of 1% or More Among Sonata 20 mg-Treated Patients | |||
Table 1 enumerates the incidence of treatment-emergent adverse events for a pool of three 28-night and one 35-night placebo-controlled studies of Sonata at doses of 5 mg or 10 mg and 20 mg. The table includes only those events that occurred in 1% or more of patients treated with Sonata 20 mg and that had a higher incidence in patients treated with Sonata 20 mg than in placebo-treated patients. | |||
The prescriber should be aware that these figures cannot be used to predict the incidence of adverse events in the course of usual medical practice where patient characteristics and other factors differ from those which prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contribution of drug and non-drug factors to the adverse event incidence rate in the population studied. | |||
[[File:Zaleplon adverse reactions t 1.png|600px|thumbnail|left]] | |||
{{clear}} | |||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
=====Body as a Whole===== | |||
=====Cardiovascular===== | |||
=====Digestive===== | |||
===== | =====Endocrine===== | ||
=====Hematologic and Lymphatic===== | |||
=====Metabolic and Nutritional===== | |||
=====Musculoskeletal===== | |||
===== | =====Neurologic===== | ||
=====Respiratory===== | |||
=====Skin and Hypersensitivy Reactions===== | |||
=====Special Senses===== | |||
=====Urogenital===== | |||
=====Miscellaneous===== | |||
<!--Drug Interactions--> | |||
|drugInteractions=As with all drugs, the potential exists for interaction with other drugs by a variety of mechanisms. | |||
CNS-Active Drugs | |||
Ethanol: Sonata 10 mg potentiated the CNS-impairing effects of ethanol 0.75 g/kg on balance testing and reaction time for 1 hour after ethanol administration and on the digit symbol substitution test (DSST), symbol copying test, and the variability component of the divided attention test for 2.5 hours after ethanol administration. The potentiation resulted from a CNS pharmacodynamic interaction; zaleplon did not affect the pharmacokinetics of ethanol. | |||
Imipramine: Coadministration of single doses of Sonata 20 mg and imipramine 75 mg produced additive effects on decreased alertness and impaired psychomotor performance for 2 to 4 hours after administration. The interaction was pharmacodynamic with no alteration of the pharmacokinetics of either drug. | |||
Paroxetine: Coadministration of a single dose of Sonata 20 mg and paroxetine 20 mg daily for 7 days did not produce any interaction on psychomotor performance. Additionally, paroxetine did not alter the pharmacokinetics of Sonata, reflecting the absence of a role of CYP2D6 in zaleplon's metabolism. | |||
Thioridazine: Coadministration of single doses of Sonata 20 mg and thioridazine 50 mg produced additive effects on decreased alertness and impaired psychomotor performance for 2 to 4 hours after administration. The interaction was pharmacodynamic with no alteration of the pharmacokinetics of either drug. | |||
Venlafaxine: Coadministration of a single dose of zaleplon 10 mg and multiple doses of venlafaxine ER (extended release) 150 mg did not result in any significant changes in the pharmacokinetics of either zaleplon or venlafaxine. In addition, there was no pharmacodynamic interaction as a result of coadministration of zaleplon and venlafaxine ER. | |||
Promethazine: Coadministration of a single dose of zaleplon and promethazine (10 and 25 mg, respectively) resulted in a 15% decrease in maximal plasma concentrations of zaleplon, but no change in the area under the plasma concentration-time curve. However, the pharmacodynamics of coadministration of zaleplon and promethazine have not been evaluated. Caution should be exercised when these 2 agents are coadministered. | |||
Drugs That Induce CYP3A4 | |||
Rifampin: CYP3A4 is ordinarily a minor metabolizing enzyme of zaleplon. Multiple-dose administration of the potent CYP3A4 inducer rifampin (600 mg every 24 hours, q24h, for 14 days), however, reduced zaleplon Cmax and AUC by approximately 80%. The coadministration of a potent CYP3A4 enzyme inducer, although not posing a safety concern, thus could lead to ineffectiveness of zaleplon. An alternative non-CYP3A4 substrate hypnotic agent may be considered in patients taking CYP3A4 inducers such as rifampin, phenytoin, carbamazepine, and phenobarbital. | |||
Drugs That Inhibit CYP3A4 | |||
CYP3A4 is a minor metabolic pathway for the elimination of zaleplon because the sum of desethylzaleplon (formed via CYP3A4 in vitro) and its metabolites, 5-oxo-desethylzaleplon and 5-oxo-desethylzaleplon glucuronide, account for only 9% of the urinary recovery of a zaleplon dose. Coadministration of single, oral doses of zaleplon with erythromycin (10 mg and 800 mg respectively), a strong, selective CYP3A4 inhibitor, produced a 34% increase in zaleplon's maximal plasma concentrations and a 20% increase in the area under the plasma concentration-time curve. The magnitude of interaction with multiple doses of erythromycin is unknown. Other strong selective CYP3A4 inhibitors such as ketoconazole can also be expected to increase the exposure of zaleplon. A routine dosage adjustment of zaleplon is not considered necessary. | |||
Drugs That Inhibit Aldehyde Oxidase | |||
The aldehyde oxidase enzyme system is less well studied than the cytochrome P450 enzyme system. | |||
Diphenhydramine: Diphenhydramine is reported to be a weak inhibitor of aldehyde oxidase in rat liver, but its inhibitory effects in human liver are not known. There is no pharmacokinetic interaction between zaleplon and diphenhydramine following the administration of a single dose (10 mg and 50 mg, respectively) of each drug. However, because both of these compounds have CNS effects, an additive pharmacodynamic effect is possible. | |||
Drugs That Inhibit Both Aldehyde Oxidase and CYP3A4 | |||
Cimetidine: Cimetidine inhibits both aldehyde oxidase (in vitro) and CYP3A4 (in vitro and in vivo), the primary and secondary enzymes, respectively, responsible for zaleplon metabolism. Concomitant administration of Sonata (10 mg) and cimetidine (800 mg) produced an 85% increase in the mean Cmax and AUC of zaleplon. An initial dose of 5 mg should be given to patients who are concomitantly being treated with cimetidine (see DOSAGE AND ADMINISTRATION). | |||
Drugs Highly Bound to Plasma Protein | |||
Zaleplon is not highly bound to plasma proteins (fraction bound 60%±15%); therefore, the disposition of zaleplon is not expected to be sensitive to alterations in protein binding. In addition, administration of Sonata to a patient taking another drug that is highly protein bound should not cause transient increase in free concentrations of the other drug. | |||
Drugs with a Narrow Therapeutic Index | |||
Digoxin: Sonata (10 mg) did not affect the pharmacokinetic or pharmacodynamic profile of digoxin (0.375 mg q24h for 8 days). | |||
Warfarin: Multiple oral doses of Sonata (20 mg q24h for 13 days) did not affect the pharmacokinetics of warfarin (R+)- or (S-)-enantiomers or the pharmacodynamics (prothrombin time) following a single 25-mg oral dose of warfarin. | |||
Drugs That Alter Renal Excretion | |||
Ibuprofen: Ibuprofen is known to affect renal function and, consequently, alter the renal excretion of other drugs. There was no apparent pharmacokinetic interaction between zaleplon and ibuprofen following single dose administration (10 mg and 600 mg, respectively) of each drug. This was expected because zaleplon is primarily metabolized and renal excretion of unchanged zaleplon accounts for less than 1% of the administered dose. | |||
|FDAPregCat=A | |||
|useInPregnancyFDA=Pregnancy: Pregnancy Category C | |||
In embryofetal development studies in rats and rabbits, oral administration of up to 100 mg/kg/day and 50 mg/kg/day, respectively, to pregnant animals throughout organogenesis produced no evidence of teratogenicity. These doses are equivalent to 49 (rat) and 48 (rabbit) times the maximum recommended human dose (MRHD) of 20 mg on a mg/m2 basis. In rats, pre- and postnatal growth was reduced in the offspring of dams receiving 100 mg/kg/day. This dose was also maternally toxic, as evidenced by clinical signs and decreased maternal body weight gain during gestation. The no-effect dose for rat offspring growth reduction was 10 mg/kg (a dose equivalent to 5 times the MRHD of 20 mg on a mg/m2 basis). No adverse effects on embryofetal development were observed in rabbits at the doses examined. | |||
In a pre- and postnatal development study in rats, increased stillbirth and postnatal mortality, and decreased growth and physical development, were observed in the offspring of females treated with doses of 7 mg/kg/day or greater during the latter part of gestation and throughout lactation. There was no evidence of maternal toxicity at this dose. The no-effect dose for offspring development was 1 mg/kg/day (a dose equivalent to 0.5 times the MRHD of 20 mg on a mg/m2 basis). When the adverse effects on offspring viability and growth were examined in a cross-fostering study, they appeared to result from both in utero and lactational exposure to the drug. | |||
There are no studies of zaleplon in pregnant women; therefore, Sonata® (zaleplon) is not recommended for use in women during pregnancy. | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | ||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | ||
|useInLaborDelivery= | |useInLaborDelivery=Sonata has no established use in labor and delivery. | ||
|useInNursing= | |useInNursing=A study in lactating mothers indicated that the clearance and half-life of zaleplon is similar to that in young normal subjects. A small amount of zaleplon is excreted in breast milk, with the highest excreted amount occurring during a feeding at approximately 1 hour after Sonata administration. Since the small amount of the drug from breast milk may result in potentially important concentrations in infants, and because the effects of zaleplon on a nursing infant are not known, it is recommended that nursing mothers not take Sonata. | ||
|useInPed= | |useInPed=The safety and effectiveness of Sonata in pediatric patients have not been established. | ||
|useInGeri= | |useInGeri=A total of 628 patients in double-blind, placebo-controlled, parallel-group clinical trials who received Sonata were at least 65 years of age; of these, 311 received 5 mg and 317 received 10 mg. In both sleep laboratory and outpatient studies, elderly patients with insomnia responded to a 5 mg dose with a reduced sleep latency, and thus 5 mg is the recommended dose in this population. During short-term treatment (14 night studies) of elderly patients with Sonata, no adverse event with a frequency of at least 1% occurred at a significantly higher rate with either 5 mg or 10 mg Sonata than with placebo. | ||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | ||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | ||
| Line 292: | Line 351: | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox= | |drugBox=[[File:Zaleplon image.png|600px|thumbnail|left]] | ||
{{clear}} | |||
|mechAction=* | |mechAction=* | ||
<!--Structure--> | <!--Structure--> | ||
|structure= | |structure=[[File:Zaleplon strucuture.png|600px|thumbnail|left]] | ||
{{clear}} | |||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | ||
| Line 307: | Line 364: | ||
<!--Nonclinical Toxicology--> | <!--Nonclinical Toxicology--> | ||
|nonClinToxic=There | |nonClinToxic=Carcinogenesis, Mutagenesis, and Impairment of Fertility | ||
Carcinogenesis | |||
Lifetime carcinogenicity studies of zaleplon were conducted in mice and rats. Mice received doses of 25 mg/kg/day, 50 mg/kg/day, 100 mg/kg/day, and 200 mg/kg/day in the diet for two years. These doses are equivalent to 6 to 49 times the maximum recommended human dose (MRHD) of 20 mg on a mg/m2 basis. There was a significant increase in the incidence of hepatocellular adenomas in female mice in the high dose group. Rats received doses of 1 mg/kg/day, 10 mg/kg/day, and 20 mg/kg/day in the diet for two years. These doses are equivalent to 0.5 to 10 times the maximum recommended human dose (MRHD) of 20 mg on a mg/m2 basis. Zaleplon was not carcinogenic in rats. | |||
Mutagenesis | |||
Zaleplon was clastogenic, both in the presence and absence of metabolic activation, causing structural and numerical aberrations (polyploidy and endoreduplication), when tested for chromosomal aberrations in the in vitro Chinese hamster ovary cell assay. In the in vitro human lymphocyte assay, zaleplon caused numerical, but not structural, aberrations only in the presence of metabolic activation at the highest concentrations tested. In other in vitro assays, zaleplon was not mutagenic in the Ames bacterial gene mutation assay or the Chinese hamster ovary HGPRT gene mutation assay. Zaleplon was not clastogenic in two in vivo assays, the mouse bone marrow micronucleus assay and the rat bone marrow chromosomal aberration assay, and did not cause DNA damage in the rat hepatocyte unscheduled DNA synthesis assay. | |||
Impairment of Fertility | |||
In a fertility and reproductive performance study in rats, mortality and decreased fertility were associated with administration of an oral dose of zaleplon of 100 mg/kg/day to males and females prior to and during mating. This dose is equivalent to 49 times the maximum recommended human dose (MRHD) of 20 mg on a mg/m2 basis. Follow-up studies indicated that impaired fertility was due to an effect on the female. | |||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | ||
| Line 316: | Line 384: | ||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
| | |packLabel=[[File:Zaleplon pdp 1.png|600px|thumbnail|left]] | ||
{{clear}} | |||
[[File:Zaleplon pdp 2.png|600px|thumbnail|left]] | |||
{{clear}} | |||
[[File:Zaleplon label.png|600px|thumbnail|left]] | |||
{{clear}} | |||
|fdaPatientInfo=Information for Patients | |||
A patient Medication Guide is also available for Sonata. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions that they may have. | |||
SPECIAL CONCERNS "Sleep-Driving" and other complex behaviors | |||
There have been reports of people getting out of bed after taking a sedative hypnotic medicine and driving their cars while not fully awake, often with no memory of the event. If a patient experiences such an episode, it should be reported to his or her doctor immediately, since "sleep-driving" can be dangerous. This behavior is more likely to occur when Sonata is taken with alcohol or other central nervous system depressants (see WARNINGS). Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sleep medicine. As with sleep-driving, patients usually do not remember these events. | |||
[[File:Zaleplon medication guide.png|600px|thumbnail|left]] | |||
{{clear}} | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
Revision as of 15:34, 18 November 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
Zaleplon is a anticonvulsant that is FDA approved for the treatment of {{{indication}}}. There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition1
- Dosing Information
- Dosage
Condition2
- Dosing Information
- Dosage
Condition3
- Dosing Information
- Dosage
Condition4
- Dosing Information
- Dosage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Zaleplon in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Zaleplon in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Zaleplon in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Zaleplon in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Zaleplon in pediatric patients.
Contraindications
- Hypersensitivity to zaleplon or any excipients in the formulation (see also PRECAUTIONS).
Warnings
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
- Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative/hypnotic drugs, including Sonata. Because some of the important adverse effects of Sonata appear to be dose-related, it is important to use the lowest possible effective dose, especially in the elderly (see DOSAGE AND ADMINISTRATION).
A variety of abnormal thinking and behavior changes have been reported to occur in association with the use of sedative/hypnotics. Some of these changes may be characterized by decreased inhibition (e.g., aggressiveness and extroversion that seem out of character), similar to effects produced by alcohol and other CNS depressants. Other reported behavioral changes have included bizarre behavior, agitation, hallucinations, and depersonalization.
Abnormal Thinking and Behavioral Changes
Complex behaviors such as "sleep-driving" (i.e., driving while not fully awake after ingestion of a sedative-hypnotic, with amnesia for the event) have been reported. These events can occur in sedative-hypnotic-naive as well as in sedative-hypnotic-experienced persons. Although behaviors such as sleep-driving may occur with Sonata alone at therapeutic doses, the use of alcohol and other CNS depressants with Sonata appears to increase the risk of such behaviors, as does the use of Sonata at doses exceeding the maximum recommended dose. Due to the risk to the patient and the community, discontinuation of Sonata should be strongly considered for patients who report a "sleep-driving" episode. Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events. Amnesia and other neuropsychiatric symptoms may occur unpredictably. In primarily depressed patients, worsening of depression, including suicidal thoughts and actions (including completed suicides), has been reported in association with the use of sedative/hypnotics.
It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above is drug induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
Following rapid dose decrease or abrupt discontinuation of the use of sedative/hypnotics, there have been reports of signs and symptoms similar to those associated with withdrawal from other CNS-depressant drugs (see DRUG ABUSE AND DEPENDENCE).
Sonata, like other hypnotics, has CNS-depressant effects. Because of the rapid onset of action, Sonata should only be ingested immediately prior to going to bed or after the patient has gone to bed and has experienced difficulty falling asleep. Patients receiving Sonata should be cautioned against engaging in hazardous occupations requiring complete mental alertness or motor coordination (e.g., operating machinery or driving a motor vehicle) after ingesting the drug, including potential impairment of the performance of such activities that may occur the day following ingestion of Sonata. Sonata, as well as other hypnotics, may produce additive CNS-depressant effects when coadministered with other psychotropic medications, anticonvulsants, antihistamines, narcotic analgesics, anesthetics, ethanol, and other drugs that themselves produce CNS depression. Sonata should not be taken with alcohol. Dosage adjustment may be necessary when Sonata is administered with other CNS-depressant agents because of the potentially additive effects.
Severe anaphylactic and anaphylactoid reactions
Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of sedative-hypnotics, including Sonata. Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis. Some patients have required medical therapy in the emergency department. If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal. Patients who develop angioedema after treatment with Sonata should not be rechallenged with the drug.
Precautions
- General
Timing of Drug Administration
Sonata should be taken immediately before bedtime or after the patient has gone to bed and has experienced difficulty falling asleep. As with all sedative/hypnotics, taking Sonata while still up and about may result in short-term memory impairment, hallucinations, impaired coordination, dizziness, and lightheadedness.
Use in the elderly and/or debilitated patients
Impaired motor and/or cognitive performance after repeated exposure or unusual sensitivity to sedative/hypnotic drugs is a concern in the treatment of elderly and/or debilitated patients. A dose of 5 mg is recommended for elderly patients to decrease the possibility of side effects (see DOSAGE AND ADMINISTRATION). Elderly and/or debilitated patients should be monitored closely.
Use in patients with concomitant illness
Clinical experience with Sonata in patients with concomitant systemic illness is limited. Sonata should be used with caution in patients with diseases or conditions that could affect metabolism or hemodynamic responses.
Although preliminary studies did not reveal respiratory depressant effects at hypnotic doses of Sonata in normal subjects, caution should be observed if Sonata (zaleplon) is prescribed to patients with compromised respiratory function, because sedative/hypnotics have the capacity to depress respiratory drive. Controlled trials of acute administration of Sonata 10 mg in patients with mild to moderate chronic obstructive pulmonary disease or moderate obstructive sleep apnea showed no evidence of alterations in blood gases or apnea/hypopnea index, respectively. However, patients with compromised respiration due to preexisting illness should be monitored carefully.
The dose of Sonata should be reduced to 5 mg in patients with mild to moderate hepatic impairment (see DOSAGE AND ADMINISTRATION). It is not recommended for use in patients with severe hepatic impairment.
No dose adjustment is necessary in patients with mild to moderate renal impairment. Sonata has not been adequately studied in patients with severe renal impairment.
Use in patients with depression
As with other sedative/hypnotic drugs, Sonata should be administered with caution to patients exhibiting signs or symptoms of depression. Suicidal tendencies may be present in such patients and protective measures may be required. Intentional overdosage is more common in this group of patients (see OVERDOSAGE); therefore, the least amount of drug that is feasible should be prescribed for the patient at any one time.
This product contains FD&C Yellow No. 5 (tartrazine) which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
Information for Patients
A patient Medication Guide is also available for Sonata. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions that they may have.
SPECIAL CONCERNS "Sleep-Driving" and other complex behaviors
There have been reports of people getting out of bed after taking a sedative hypnotic medicine and driving their cars while not fully awake, often with no memory of the event. If a patient experiences such an episode, it should be reported to his or her doctor immediately, since "sleep-driving" can be dangerous. This behavior is more likely to occur when Sonata is taken with alcohol or other central nervous system depressants (see WARNINGS). Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sleep medicine. As with sleep-driving, patients usually do not remember these events.
Laboratory Tests
There are no specific laboratory tests recommended.
Adverse Reactions
Clinical Trials Experience
The premarketing development program for Sonata included zaleplon exposures in patients and/or normal subjects from 2 different groups of studies: approximately 900 normal subjects in clinical pharmacology/pharmacokinetic studies; and approximately 2,900 exposures from patients in placebo-controlled clinical effectiveness studies, corresponding to approximately 450 patient exposure years. The conditions and duration of treatment with Sonata varied greatly and included (in overlapping categories) open-label and double-blind phases of studies, inpatients and outpatients, and short-term or longer-term exposure. Adverse reactions were assessed by collecting adverse events, results of physical examinations, vital signs, weights, laboratory analyses, and ECGs.
Adverse events during exposure were obtained primarily by general inquiry and recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse events without first grouping similar types of events into a smaller number of standardized event categories. In the tables and tabulations that follow, COSTART terminology has been used to classify reported adverse events.
The stated frequencies of adverse events represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse event of the type listed. An event was considered treatment-emergent if it occurred for the first time or worsened while receiving therapy following baseline evaluation.
Adverse Findings Observed in Short-Term, Placebo-Controlled Trials
Adverse Events Associated With Discontinuation of Treatment
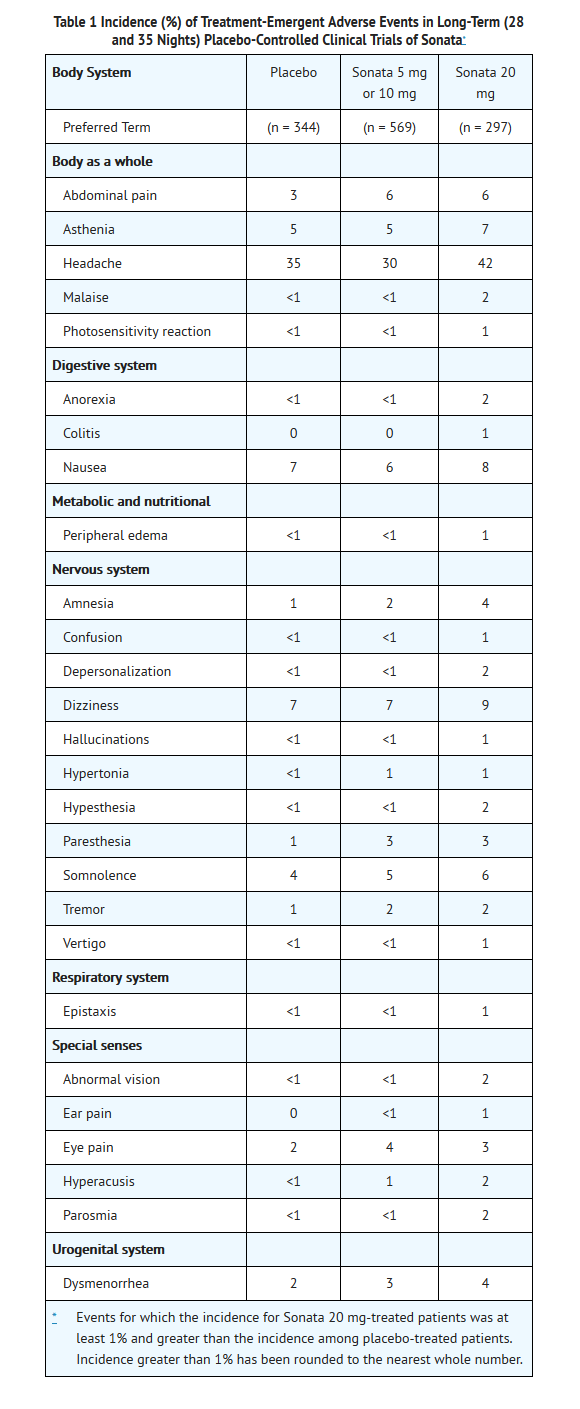
In premarketing placebo-controlled, parallel-group phase 2 and phase 3 clinical trials, 3.1% of 744 patients who received placebo and 3.7% of 2,149 patients who received Sonata discontinued treatment because of an adverse clinical event. This difference was not statistically significant. No event that resulted in discontinuation occurred at a rate of ≥ 1%.
Adverse Events Occurring at an Incidence of 1% or More Among Sonata 20 mg-Treated Patients
Table 1 enumerates the incidence of treatment-emergent adverse events for a pool of three 28-night and one 35-night placebo-controlled studies of Sonata at doses of 5 mg or 10 mg and 20 mg. The table includes only those events that occurred in 1% or more of patients treated with Sonata 20 mg and that had a higher incidence in patients treated with Sonata 20 mg than in placebo-treated patients.
The prescriber should be aware that these figures cannot be used to predict the incidence of adverse events in the course of usual medical practice where patient characteristics and other factors differ from those which prevailed in the clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigations involving different treatments, uses, and investigators. The cited figures, however, do provide the prescribing physician with some basis for estimating the relative contribution of drug and non-drug factors to the adverse event incidence rate in the population studied.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Zaleplon in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
As with all drugs, the potential exists for interaction with other drugs by a variety of mechanisms.
CNS-Active Drugs
Ethanol: Sonata 10 mg potentiated the CNS-impairing effects of ethanol 0.75 g/kg on balance testing and reaction time for 1 hour after ethanol administration and on the digit symbol substitution test (DSST), symbol copying test, and the variability component of the divided attention test for 2.5 hours after ethanol administration. The potentiation resulted from a CNS pharmacodynamic interaction; zaleplon did not affect the pharmacokinetics of ethanol.
Imipramine: Coadministration of single doses of Sonata 20 mg and imipramine 75 mg produced additive effects on decreased alertness and impaired psychomotor performance for 2 to 4 hours after administration. The interaction was pharmacodynamic with no alteration of the pharmacokinetics of either drug.
Paroxetine: Coadministration of a single dose of Sonata 20 mg and paroxetine 20 mg daily for 7 days did not produce any interaction on psychomotor performance. Additionally, paroxetine did not alter the pharmacokinetics of Sonata, reflecting the absence of a role of CYP2D6 in zaleplon's metabolism.
Thioridazine: Coadministration of single doses of Sonata 20 mg and thioridazine 50 mg produced additive effects on decreased alertness and impaired psychomotor performance for 2 to 4 hours after administration. The interaction was pharmacodynamic with no alteration of the pharmacokinetics of either drug.
Venlafaxine: Coadministration of a single dose of zaleplon 10 mg and multiple doses of venlafaxine ER (extended release) 150 mg did not result in any significant changes in the pharmacokinetics of either zaleplon or venlafaxine. In addition, there was no pharmacodynamic interaction as a result of coadministration of zaleplon and venlafaxine ER.
Promethazine: Coadministration of a single dose of zaleplon and promethazine (10 and 25 mg, respectively) resulted in a 15% decrease in maximal plasma concentrations of zaleplon, but no change in the area under the plasma concentration-time curve. However, the pharmacodynamics of coadministration of zaleplon and promethazine have not been evaluated. Caution should be exercised when these 2 agents are coadministered.
Drugs That Induce CYP3A4
Rifampin: CYP3A4 is ordinarily a minor metabolizing enzyme of zaleplon. Multiple-dose administration of the potent CYP3A4 inducer rifampin (600 mg every 24 hours, q24h, for 14 days), however, reduced zaleplon Cmax and AUC by approximately 80%. The coadministration of a potent CYP3A4 enzyme inducer, although not posing a safety concern, thus could lead to ineffectiveness of zaleplon. An alternative non-CYP3A4 substrate hypnotic agent may be considered in patients taking CYP3A4 inducers such as rifampin, phenytoin, carbamazepine, and phenobarbital.
Drugs That Inhibit CYP3A4
CYP3A4 is a minor metabolic pathway for the elimination of zaleplon because the sum of desethylzaleplon (formed via CYP3A4 in vitro) and its metabolites, 5-oxo-desethylzaleplon and 5-oxo-desethylzaleplon glucuronide, account for only 9% of the urinary recovery of a zaleplon dose. Coadministration of single, oral doses of zaleplon with erythromycin (10 mg and 800 mg respectively), a strong, selective CYP3A4 inhibitor, produced a 34% increase in zaleplon's maximal plasma concentrations and a 20% increase in the area under the plasma concentration-time curve. The magnitude of interaction with multiple doses of erythromycin is unknown. Other strong selective CYP3A4 inhibitors such as ketoconazole can also be expected to increase the exposure of zaleplon. A routine dosage adjustment of zaleplon is not considered necessary.
Drugs That Inhibit Aldehyde Oxidase
The aldehyde oxidase enzyme system is less well studied than the cytochrome P450 enzyme system.
Diphenhydramine: Diphenhydramine is reported to be a weak inhibitor of aldehyde oxidase in rat liver, but its inhibitory effects in human liver are not known. There is no pharmacokinetic interaction between zaleplon and diphenhydramine following the administration of a single dose (10 mg and 50 mg, respectively) of each drug. However, because both of these compounds have CNS effects, an additive pharmacodynamic effect is possible.
Drugs That Inhibit Both Aldehyde Oxidase and CYP3A4
Cimetidine: Cimetidine inhibits both aldehyde oxidase (in vitro) and CYP3A4 (in vitro and in vivo), the primary and secondary enzymes, respectively, responsible for zaleplon metabolism. Concomitant administration of Sonata (10 mg) and cimetidine (800 mg) produced an 85% increase in the mean Cmax and AUC of zaleplon. An initial dose of 5 mg should be given to patients who are concomitantly being treated with cimetidine (see DOSAGE AND ADMINISTRATION).
Drugs Highly Bound to Plasma Protein
Zaleplon is not highly bound to plasma proteins (fraction bound 60%±15%); therefore, the disposition of zaleplon is not expected to be sensitive to alterations in protein binding. In addition, administration of Sonata to a patient taking another drug that is highly protein bound should not cause transient increase in free concentrations of the other drug.
Drugs with a Narrow Therapeutic Index
Digoxin: Sonata (10 mg) did not affect the pharmacokinetic or pharmacodynamic profile of digoxin (0.375 mg q24h for 8 days).
Warfarin: Multiple oral doses of Sonata (20 mg q24h for 13 days) did not affect the pharmacokinetics of warfarin (R+)- or (S-)-enantiomers or the pharmacodynamics (prothrombin time) following a single 25-mg oral dose of warfarin.
Drugs That Alter Renal Excretion
Ibuprofen: Ibuprofen is known to affect renal function and, consequently, alter the renal excretion of other drugs. There was no apparent pharmacokinetic interaction between zaleplon and ibuprofen following single dose administration (10 mg and 600 mg, respectively) of each drug. This was expected because zaleplon is primarily metabolized and renal excretion of unchanged zaleplon accounts for less than 1% of the administered dose.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): A Pregnancy: Pregnancy Category C
In embryofetal development studies in rats and rabbits, oral administration of up to 100 mg/kg/day and 50 mg/kg/day, respectively, to pregnant animals throughout organogenesis produced no evidence of teratogenicity. These doses are equivalent to 49 (rat) and 48 (rabbit) times the maximum recommended human dose (MRHD) of 20 mg on a mg/m2 basis. In rats, pre- and postnatal growth was reduced in the offspring of dams receiving 100 mg/kg/day. This dose was also maternally toxic, as evidenced by clinical signs and decreased maternal body weight gain during gestation. The no-effect dose for rat offspring growth reduction was 10 mg/kg (a dose equivalent to 5 times the MRHD of 20 mg on a mg/m2 basis). No adverse effects on embryofetal development were observed in rabbits at the doses examined.
In a pre- and postnatal development study in rats, increased stillbirth and postnatal mortality, and decreased growth and physical development, were observed in the offspring of females treated with doses of 7 mg/kg/day or greater during the latter part of gestation and throughout lactation. There was no evidence of maternal toxicity at this dose. The no-effect dose for offspring development was 1 mg/kg/day (a dose equivalent to 0.5 times the MRHD of 20 mg on a mg/m2 basis). When the adverse effects on offspring viability and growth were examined in a cross-fostering study, they appeared to result from both in utero and lactational exposure to the drug.
There are no studies of zaleplon in pregnant women; therefore, Sonata® (zaleplon) is not recommended for use in women during pregnancy.
Pregnancy Category (AUS):
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Zaleplon in women who are pregnant.
Labor and Delivery
Sonata has no established use in labor and delivery.
Nursing Mothers
A study in lactating mothers indicated that the clearance and half-life of zaleplon is similar to that in young normal subjects. A small amount of zaleplon is excreted in breast milk, with the highest excreted amount occurring during a feeding at approximately 1 hour after Sonata administration. Since the small amount of the drug from breast milk may result in potentially important concentrations in infants, and because the effects of zaleplon on a nursing infant are not known, it is recommended that nursing mothers not take Sonata.
Pediatric Use
The safety and effectiveness of Sonata in pediatric patients have not been established.
Geriatic Use
A total of 628 patients in double-blind, placebo-controlled, parallel-group clinical trials who received Sonata were at least 65 years of age; of these, 311 received 5 mg and 317 received 10 mg. In both sleep laboratory and outpatient studies, elderly patients with insomnia responded to a 5 mg dose with a reduced sleep latency, and thus 5 mg is the recommended dose in this population. During short-term treatment (14 night studies) of elderly patients with Sonata, no adverse event with a frequency of at least 1% occurred at a significantly higher rate with either 5 mg or 10 mg Sonata than with placebo.
Gender
There is no FDA guidance on the use of Zaleplon with respect to specific gender populations.
Race
There is no FDA guidance on the use of Zaleplon with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Zaleplon in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Zaleplon in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Zaleplon in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Zaleplon in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Zaleplon in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Zaleplon in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Zaleplon in the drug label.
Pharmacology
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Zaleplon in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Zaleplon in the drug label.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Carcinogenesis
Lifetime carcinogenicity studies of zaleplon were conducted in mice and rats. Mice received doses of 25 mg/kg/day, 50 mg/kg/day, 100 mg/kg/day, and 200 mg/kg/day in the diet for two years. These doses are equivalent to 6 to 49 times the maximum recommended human dose (MRHD) of 20 mg on a mg/m2 basis. There was a significant increase in the incidence of hepatocellular adenomas in female mice in the high dose group. Rats received doses of 1 mg/kg/day, 10 mg/kg/day, and 20 mg/kg/day in the diet for two years. These doses are equivalent to 0.5 to 10 times the maximum recommended human dose (MRHD) of 20 mg on a mg/m2 basis. Zaleplon was not carcinogenic in rats.
Mutagenesis
Zaleplon was clastogenic, both in the presence and absence of metabolic activation, causing structural and numerical aberrations (polyploidy and endoreduplication), when tested for chromosomal aberrations in the in vitro Chinese hamster ovary cell assay. In the in vitro human lymphocyte assay, zaleplon caused numerical, but not structural, aberrations only in the presence of metabolic activation at the highest concentrations tested. In other in vitro assays, zaleplon was not mutagenic in the Ames bacterial gene mutation assay or the Chinese hamster ovary HGPRT gene mutation assay. Zaleplon was not clastogenic in two in vivo assays, the mouse bone marrow micronucleus assay and the rat bone marrow chromosomal aberration assay, and did not cause DNA damage in the rat hepatocyte unscheduled DNA synthesis assay.
Impairment of Fertility
In a fertility and reproductive performance study in rats, mortality and decreased fertility were associated with administration of an oral dose of zaleplon of 100 mg/kg/day to males and females prior to and during mating. This dose is equivalent to 49 times the maximum recommended human dose (MRHD) of 20 mg on a mg/m2 basis. Follow-up studies indicated that impaired fertility was due to an effect on the female.
Clinical Studies
There is limited information regarding Clinical Studies of Zaleplon in the drug label.
How Supplied
Storage
There is limited information regarding Zaleplon Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Zaleplon |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
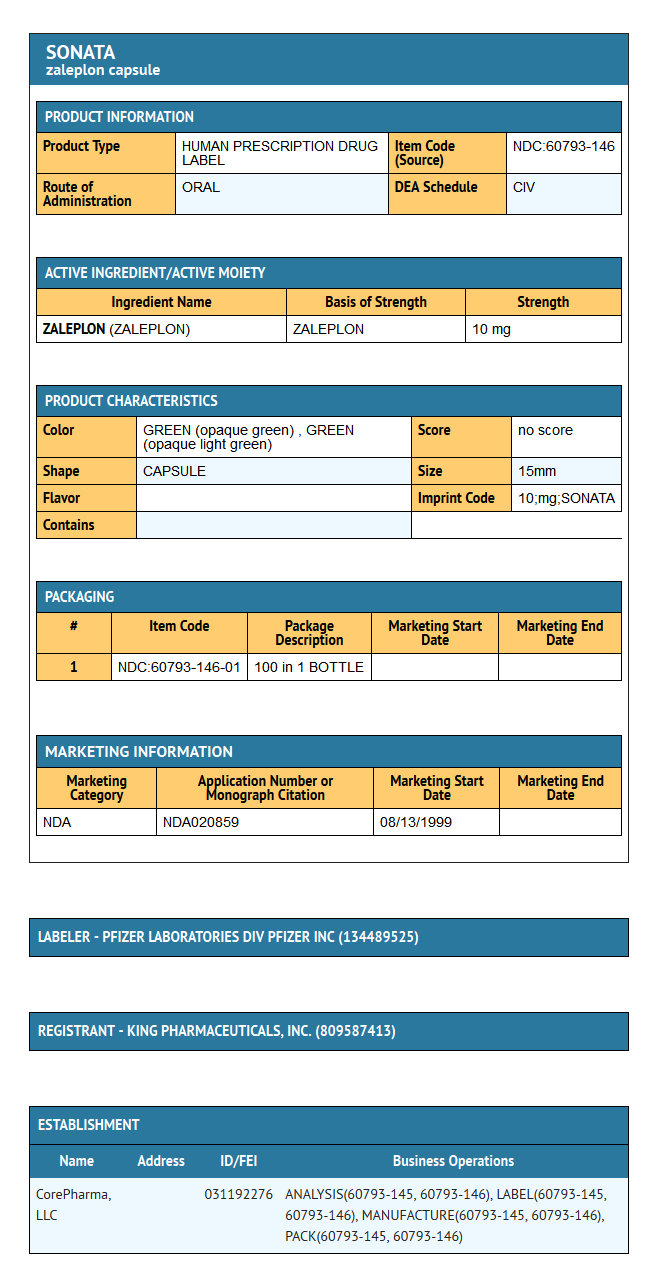
{{#ask: Label Page::Zaleplon |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information for Patients
A patient Medication Guide is also available for Sonata. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions that they may have.
SPECIAL CONCERNS "Sleep-Driving" and other complex behaviors
There have been reports of people getting out of bed after taking a sedative hypnotic medicine and driving their cars while not fully awake, often with no memory of the event. If a patient experiences such an episode, it should be reported to his or her doctor immediately, since "sleep-driving" can be dangerous. This behavior is more likely to occur when Sonata is taken with alcohol or other central nervous system depressants (see WARNINGS). Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sleep medicine. As with sleep-driving, patients usually do not remember these events.

Precautions with Alcohol
- Alcohol-Zaleplon interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Zaleplon
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Zaleplon |Label Name=Zaleplon11.png
}}
{{#subobject:
|Label Page=Zaleplon |Label Name=Zaleplon11.png
}}