Venetoclax
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Martin Nino, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Venetoclax is a BCL-2 inhibitor that is FDA approved for the treatment of patients with chronic lymphocytic leukemia (CLL) with 17p deletion, as detected by an FDA approved test, who have received at least one prior therapy. This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial. Common adverse reactions include neutropenia, diarrhea, nausea, anemia, upper respiratory tract infection, thrombocytopenia, and fatigue (≥20%).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Patient Selection
Select patients for the treatment of relapsed or refractory CLL with Venetoclax based on the presence of 17p deletions in blood specimens. Patients without 17p deletion at diagnosis should be retested at relapse because acquisition of 17p deletion can occur.
Dosage
Venetoclax should be taken orally once daily until disease progression or unacceptable toxicity is observed. Assess patient-specific factors for level of risk of tumor lysis syndrome (TLS) and provide prophylactic hydration and anti-hyperuricemics to patients prior to first dose of Venetoclax to reduce risk of TLS. Administer the Venetoclax dose according to a weekly ramp-up schedule over 5 weeks to the recommended daily dose of 400 mg as shown in Table 1. The 5-week ramp-up dosing schedule is designed to gradually reduce tumor burden (debulk) and decrease the risk of TLS. Once the ramp-up phase is completed, the 400 mg dose is achieved using 100 mg tablets.
- Table 1: Dosing Schedule for Ramp-Up Phase

Instruct patients to take Venetoclax tablets with a meal and water at approximately the same time each day. Venetoclax tablets should be swallowed whole and not chewed, crushed, or broken prior to swallowing.
Risk Assessment and Prophylaxis for Tumor Lysis Syndrome
Venetoclax can cause rapid reduction in tumor and thus poses a risk for TLS in the initial 5-week ramp-up phase. Changes in blood chemistries consistent with TLS that require prompt management can occur as early as 6 to 8 hours following the first dose of VENCLEXTA and at each dose increase.
The risk of TLS is a continuum based on multiple factors, including tumor burden and comorbidities. Perform tumor burden assessments, including radiographic evaluation (e.g., CT scan), assess blood chemistry (potassium, uric acid, phosphorus, calcium, and creatinine) in all patients and correct pre-existing abnormalities prior to initiation of treatment with Venetoclax. Reduced renal function (creatinine clearance [CrCl] <80 mL/min) further increases the risk. The risk may decrease as tumor burden decreases.
Table 2 below describes the recommended TLS prophylaxis and monitoring during Venetoclax treatment based on tumor burden determination from clinical trial data.
- Table 2: Recommended TLS Prophylaxis Based on Tumor Burden From Clinical Trial Data (consider all patient co-morbidities before final determination of prophylaxis and monitoring schedule)

Dose Modifications Based on Toxicities
Interrupt dosing or reduce dose for toxicities. See Table 3 for dose modifications for hematologic and other toxicities related to Venetoclax, and Table 4 for dose. For patients who have had a dosing interruption greater than 1 week during the first 5 weeks of ramp-up phase or greater than 2 weeks when at the daily dose of 400 mg, reassess for risk of TLS to determine if reinitiation with a reduced dose is necessary (e.g., all or some levels of the dose ramp-up schedule).
- Table 3: Recommended Dose Modifications for Toxicities (a)

- Table 4: Dose Modification for Toxicity During Venetoclax Treatment

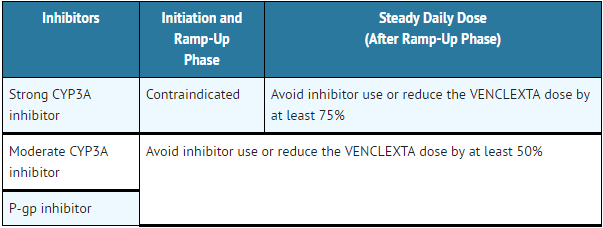
Dose Modifications for Use with CYP3A and P-gp Inhibitors
Concomitant use of Venetoclax with strong CYP3A inhibitors at initiation and during ramp-up phase is contraindicated. Concomitant use of Venetoclax with strong CYP3A inhibitors increases Venetoclax exposure (i.e., Cmax and AUC) and may increase the risk for TLS at initiation and during ramp-up phase. For patients who have completed the ramp-up phase and are on a steady daily dose of Venetoclax, reduce the Venetoclax dose by at least 75% when strong CYP3A inhibitors must be used concomitantly.
Avoid concomitant use of Venetoclax with moderate CYP3A inhibitors or P-gp inhibitors. Consider alternative treatments. If a moderate CYP3A inhibitor or a P-gp inhibitor must be used, reduce the Venetoclax dose by at least 50%. Monitor these patients more closely for signs of toxicities.
Resume the Venetoclax dose that was used prior to initiating the CYP3A inhibitor or P-gp inhibitor 2 to 3 days after discontinuation of the inhibitor.
The recommendations for managing drug-drug interactions are summarized in Table 5.
- Table 5: Management of Potential Venetoclax Interactions with CYP3A and P-gp Inhibitors
Missed Dose
If the patient misses a dose of Venetoclax within 8 hours of the time it is usually taken, the patient should take the missed dose as soon as possible and resume the normal daily dosing schedule. If a patient misses a dose by more than 8 hours, the patient should not take the missed dose and should resume the usual dosing schedule the next day.
If the patient vomits following dosing, no additional dose should be taken that day. The next prescribed dose should be taken at the usual time.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Venetoclax in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Venetoclax in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness have not been established in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Venetoclax in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Venetoclax in pediatric patients.
Contraindications
Concomitant use of Venetoclax with strong CYP3A inhibitors at initiation and during ramp-up phase is contraindicated.
Warnings
Tumor Lysis Syndrome
Tumor lysis syndrome, including fatal events and renal failure requiring dialysis, has occurred in previously treated CLL patients with high tumor burden when treated with Venetoclax.
Venetoclax can cause rapid reduction in tumor and thus poses a risk for TLS in the initial 5-week ramp-up phase. Changes in blood chemistries consistent with TLS that require prompt management can occur as early as 6 to 8 hours following the first dose of Venetoclax and at each dose increase.
The risk of TLS is a continuum based on multiple factors, including tumor burden (see TABLE 2) and comorbidities. Reduced renal function (CrCl <80 mL/min) further increases the risk. Patients should be assessed for risk and should receive appropriate prophylaxis for TLS, including hydration and anti-hyperuricemics. Monitor blood chemistries and manage abnormalities promptly. Interrupt dosing if needed. Employ more intensive measures (intravenous hydration, frequent monitoring, hospitalization) as overall risk increases.
Concomitant use of Venetoclax with strong or moderate CYP3A inhibitors and P-gp inhibitors increases venetoclax exposure, may increase the risk of TLS at initiation and during ramp-up phase and may require Venetoclax dose adjustment.
Neutropenia
Grade 3 or 4 neutropenia occurred in 41% (98/240) of patients treated with Venetoclax. Monitor complete blood counts throughout the treatment period. Interrupt dosing or reduce dose for severe neutropenia. Consider supportive measures including antimicrobials for signs of infection and use of growth factors (e.g., G-CSF).
Immunization
Do not administer live attenuated vaccines prior to, during, or after treatment with Venetoclax until B-cell recovery occurs. The safety and efficacy of immunization with live attenuated vaccines during or following Venetoclax therapy have not been studied. Advise patients that vaccinations may be less effective.
Embryo-Fetal Toxicity
Based on its mechanism of action and findings in animals, Venetoclax may cause embryo-fetal harm when administered to a pregnant woman. In an embryo-fetal study conducted in mice, administration of Venetoclax to pregnant animals at exposures equivalent to that observed in patients at the recommended dose of 400 mg daily resulted in post-implantation loss and decreased fetal weight. There are no adequate and well-controlled studies in pregnant woman using Venetoclax. Advise females of reproductive potential to avoid pregnancy during treatment. If Venetoclax is used during pregnancy or if the patient becomes pregnant while taking Venetoclax, the patient should be apprised of the potential hazard to the fetus.
Adverse Reactions
Clinical Trials Experience
The following adverse drug reactions are discussed in greater detail in other sections of the label:
- Tumor Lysis Syndrome
- Neutropenia
Because clinical trials are conducted under widely variable conditions, adverse event rates observed in clinical trials of a drug cannot be directly compared with rates of clinical trials of another drug and may not reflect the rates observed in practice.
The safety of single agent Venetoclax at the 400 mg recommended daily dose following a dose ramp-up schedule is based on pooled data of 240 patients with previously treated CLL from two phase 2 trials and one phase 1 trial. In the pooled dataset, the median age was 66 years (range: 29 to 85 years), 95% were white, and 69% were male. The median number of prior therapies was 3 (range: 1 to 12). The median duration of treatment with Venetoclax at the time of data analysis was approximately 10.3 months (range: 0 to 34.1 months). Approximately 46% of patients received Venetoclax for more than 48 weeks.
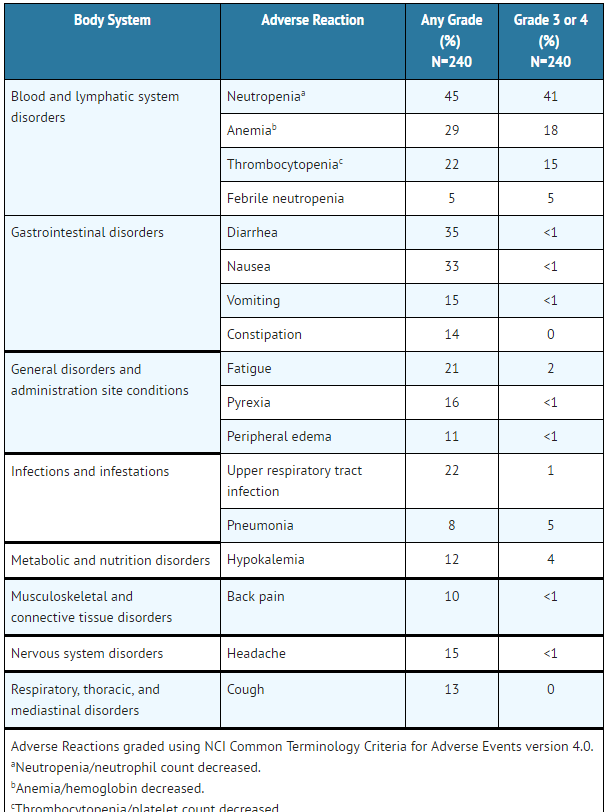
The most common adverse reactions (≥20%) of any grade were neutropenia, diarrhea, nausea, anemia, upper respiratory tract infection, thrombocytopenia, and fatigue.
Serious adverse reactions were reported in 43.8% of patients. The most frequent serious adverse reactions (≥2%) were pneumonia, febrile neutropenia, pyrexia, autoimmune hemolytic anemia (AIHA), anemia, and TLS.
Discontinuations due to adverse reactions occurred in 8.3% of patients. The most frequent adverse reactions leading to drug discontinuation were thrombocytopenia and AIHA.
Dosage adjustments due to adverse reactions occurred in 9.6% of patients. The most frequent adverse reactions leading to dose adjustments were neutropenia, febrile neutropenia, and thrombocytopenia.
Adverse reactions reported in 3 trials of patients with previously treated CLL using single agent Venetoclax are presented in Table 6.
- Table 6: Adverse Reactions Reported in ≥10% (Any Grade) or ≥5% (Grade 3 or 4) of Patients with CLL
Tumor Lysis Syndrome
Tumor lysis syndrome is an important identified risk when initiating Venetoclax. In the initial Phase 1 dose-finding trials, which had shorter (2-3 week) ramp-up phase and higher starting dose, the incidence of TLS was 12% (9/77; 4 laboratory TLS, 5 clinical TLS), including 2 fatal events and 3 events of acute renal failure, 1 requiring dialysis.
The risk of TLS was reduced after revision of the dosing regimen and modification to prophylaxis and monitoring measures. In Venetoclax clinical trials, patients with any measurable lymph node ≥10 cm or those with both an ALC ≥25 x 109/L and any measurable lymph node ≥5 cm were hospitalized to enable more intensive hydration and monitoring for the first day of dosing at 20 mg and 50 mg during the ramp-up phase.
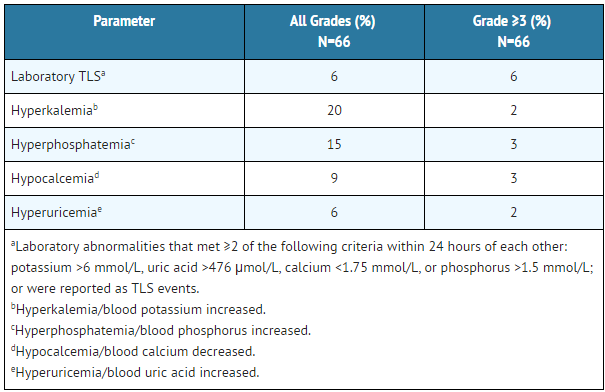
In 66 patients with CLL starting with a daily dose of 20 mg and increasing over 5 weeks to a daily dose of 400 mg, the rate of TLS was 6%. All events either met laboratory TLS criteria (laboratory abnormalities that met ≥2 of the following within 24 hours of each other: potassium >6 mmol/L, uric acid >476 µmol/L, calcium <1.75 mmol/L, or phosphorus >1.5 mmol/L); or were reported as TLS events. The events occurred in patients who had a lymph node(s) ≥5 cm or ALC ≥25 x 109/L. No TLS with clinical consequences such as acute renal failure, cardiac arrhythmias or sudden death and/or seizures was observed in these patients. All patients had CrCl ≥50 mL/min.
Laboratory abnormalities relevant to TLS observed in 66 patients with CLL who followed the dose ramp-up schedule and TLS prophylaxis measures are presented in Table 7.
- Table 7: Adverse Reactions of TLS and Relevant Laboratory Abnormalities Reported in Patients with CLL
Postmarketing Experience
There is limited information regarding Venetoclax Postmarketing Experience in the drug label.
Drug Interactions
Effects of Other Drugs on Venetoclax
Venetoclax is predominantly metabolized by CYP3A4/5.
- Strong CYP3A Inhibitors
Concomitant use of Venetoclax with strong CYP3A inhibitors (e.g., ketoconazole, conivaptan, clarithromycin, indinavir, itraconazole, lopinavir, ritonavir, telaprevir, posaconazole and voriconazole) at initiation and during ramp-up phase is contraindicated.
For patients who have completed the ramp-up phase and are on a steady daily dose of Venetoclax, reduce the Venetoclax dose by at least 75% when used concomitantly with strong CYP3A inhibitors. Resume the Venetoclax dose that was used prior to initiating the CYP3A inhibitor 2 to 3 days after discontinuation of the inhibitor.
Co-administration of ketoconazole increased Venetoclax Cmax by 2.3-fold and AUC∞ by 6.4-fold.
- Moderate CYP3A Inhibitors and P-gp Inhibitors
Avoid concomitant use of moderate CYP3A inhibitors (e.g., erythromycin, ciprofloxacin, diltiazem, dronedarone, fluconazole, verapamil) or P-gp inhibitors (e.g., amiodarone, azithromycin, captopril, carvedilol, cyclosporine, felodipine, quercetin, quinidine, ranolazine, ticagrelor) with Venetoclax. Consider alternative treatments. If a moderate CYP3A inhibitor or a P-gp inhibitor must be used, reduce the Venetoclax dose by at least 50%. Monitor patients more closely for signs of Venetoclax toxicities.
Resume the Venetoclax dose that was used prior to initiating the CYP3A inhibitor or P-gp inhibitor 2 to 3 days after discontinuation of the inhibitor.
Avoid grapefruit products, Seville oranges, and starfruit during treatment with Venetoclax, as they contain inhibitors of CYP3A.
Co-administration of a single dose of rifampin, a P-gp inhibitor, increased Venetoclax Cmax by 106% and AUC∞ by 78%.
- CYP3A Inducers
Avoid concomitant use of Venetoclax with strong CYP3A inducers (e.g., carbamazepine, phenytoin, rifampin, St. John’s wort) or moderate CYP3A inducers (e.g., bosentan, efavirenz, etravirine, modafinil, nafcillin). Consider alternative treatments with less CYP3A induction [see Clinical Pharmacology (12.3)].
Co-administration of multiple doses of rifampin, a strong CYP3A inducer, decreased Venetoclax Cmax by 42% and AUC∞ by 71%.
Effects of Venetoclax on Other Drugs
- Warfarin
In a drug-drug interaction study in healthy subjects, administration of a single dose of Venetoclax with warfarin resulted in an 18% to 28% increase in Cmax and AUC∞ of R-warfarin and S-warfarin. Because Venetoclax was not dosed to steady state, it is recommended that the international normalized ratio (INR) be monitored closely in patients receiving warfarin.
- P-gp substrates
In vitro data suggest Venetoclax has inhibition potential on P-gp substrates at therapeutic dose levels in the gut. Therefore, co-administration of narrow therapeutic index P-gp substrates (e.g., digoxin, everolimus, and sirolimus) with Venetoclax should be avoided. If a narrow therapeutic index P-gp substrate must be used, it should be taken at least 6 hours before Venetoclax.
Use in Specific Populations
Pregnancy
- Risk Summary
There are no available human data on the use of Venetoclax in pregnant women. Based on toxicity observed in mice, Venetoclax may cause fetal harm when administered to pregnant women. In mice, Venetoclax was fetotoxic at exposures 1.2 times the human clinical exposure based on AUC at the recommended human dose of 400 mg daily. If Venetoclax is used during pregnancy or if the patient becomes pregnant while taking Venetoclax, the patient should be apprised of the potential risk to a fetus.
The background risk in the U.S. general population of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies.
- Data
- Animal Data
In embryo-fetal development studies, Venetoclax was administered to pregnant mice and rabbits during the period of organogenesis. In mice, Venetoclax was associated with increased post-implantation loss and decreased fetal body weight at 150 mg/kg/day (maternal exposures approximately 1.2 times the human AUC exposure at the recommended dose of 400 mg daily). No teratogenicity was observed in either the mouse or the rabbit.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Venetoclax in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Venetoclax during labor and delivery.
Nursing Mothers
There are no data on the presence of Venetoclax in human milk, the effects of Venetoclax on the breastfed child, or the effects of Venetoclax on milk production. Because many drugs are excreted in human milk and because the potential for serious adverse reactions in breastfed infants from Venetoclax is unknown, advise nursing women to discontinue breastfeeding during treatment with Venetoclax.
Pediatric Use
Safety and effectiveness have not been established in pediatric patients.
Geriatic Use
Of the 106 patients with previously treated CLL with 17p deletion who were evaluated for efficacy, 57% were ≥65 years of age and 17% were ≥75 years of age. Of the 240 patients with previously treated CLL evaluated for safety from 3 open-label trials, 58% were ≥65 years of age and 17% were ≥75 years of age.
No overall differences in safety and effectiveness were observed between older and younger patients.
Gender
There is no FDA guidance on the use of Venetoclax with respect to specific gender populations.
Race
There is no FDA guidance on the use of Venetoclax with respect to specific racial populations.
Renal Impairment
Patients with reduced renal function (CrCl <80 mL/min) are at increased risk of TLS. These patients may require more intensive prophylaxis and monitoring to reduce the risk of TLS when initiating treatment with Venetoclax.
No specific clinical trials have been conducted in subjects with renal impairment. Less than 0.1% of radioactive Venetoclax dose was detected in urine. No dose adjustment is needed for patients with mild or moderate renal impairment (CrCl ≥30 mL/min) based on results of the population pharmacokinetic analysis. A recommended dose has not been determined for patients with severe renal impairment (CrCl <30 mL/min) or patients on dialysis.
Hepatic Impairment
No specific clinical trials have been conducted in subjects with hepatic impairment, however human mass balance study showed that Venetoclax undergoes hepatic elimination. Although no dose adjustment is recommended in patients with mild or moderate hepatic impairment based on results of the population pharmacokinetic analysis, a trend for increased adverse events was observed in patients with moderate hepatic impairment; monitor these patients more closely for signs of toxicity during the initiation and dose ramp-up phase. A recommended dose has not been determined for patients with severe hepatic impairment.
Females of Reproductive Potential and Males
Venetoclax may cause fetal harm.
- Pregnancy Testing
Females of reproductive potential should undergo pregnancy testing before initiation of Venetoclax.
- Contraception
Advise females of reproductive potential to use effective contraception during treatment with Venetoclax and for at least 30 days after the last dose.
- Infertility
Based on findings in animals, male fertility may be compromised by treatment with Venetoclax.
Immunocompromised Patients
There is no FDA guidance one the use of Venetoclax in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Venetoclax Administration in the drug label.
Monitoring
There is limited information regarding Venetoclax Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Venetoclax and IV administrations.
Overdosage
There is limited information regarding Venetoclax overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Venetoclax Pharmacology in the drug label.
Mechanism of Action
There is limited information regarding Venetoclax Mechanism of Action in the drug label.
Structure
There is limited information regarding Venetoclax Structure in the drug label.
Pharmacodynamics
There is limited information regarding Venetoclax Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Venetoclax Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Venetoclax Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Venetoclax Clinical Studies in the drug label.
How Supplied
There is limited information regarding Venetoclax How Supplied in the drug label.
Storage
There is limited information regarding Venetoclax Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Venetoclax |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Venetoclax |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Venetoclax Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Venetoclax interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Venetoclax Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Venetoclax Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.