Valnoctamide
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 94%[1] |
| Metabolism | Hepatic |
| Elimination half-life | 10 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
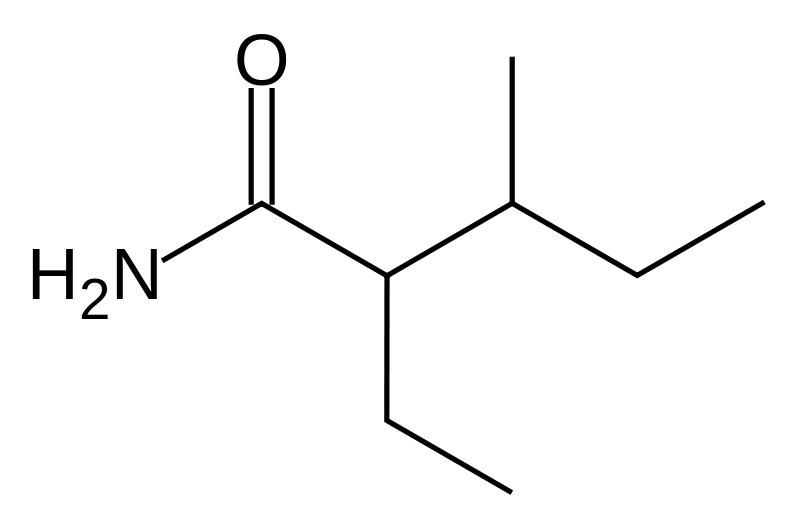
| Formula | C8H17NO |
| Molar mass | 143.227 g/mol |
|
WikiDoc Resources for Valnoctamide |
|
Articles |
|---|
|
Most recent articles on Valnoctamide Most cited articles on Valnoctamide |
|
Media |
|
Powerpoint slides on Valnoctamide |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Valnoctamide at Clinical Trials.gov Clinical Trials on Valnoctamide at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Valnoctamide
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Valnoctamide Discussion groups on Valnoctamide Patient Handouts on Valnoctamide Directions to Hospitals Treating Valnoctamide Risk calculators and risk factors for Valnoctamide
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Valnoctamide |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Valnoctamide (INN, USAN) has been used in France as an sedative-hypnotic since 1964.[2] It is a structural isomer of valpromide, a valproic acid prodrug; unlike valpromide, however, valnoctamide is not transformed into its homologous acid, valnoctic acid, in vivo.[3]
Indications
In addition to being a sedative, valnoctamide has been investigated for use in epilepsy since 1969[4] and was still being investigated in 2000[5] and 2003.
It was studied for neuropathic pain in 2005 by Winkler et al, with good results: it had minimal effects on motor coordination and alertness at effective doses, and appeared to be equally effective as gabapentin.[6]
RH Belmaker, Yuly Bersudsky and Alex Mishory started a clinical trial of valnoctamide for prophylaxis of mania in lieu of the much more teratogenic valproic acid or its salts.[7]
Side effects
The side effects of valnoctamide are mostly minor and include somnolence[8] and the slight motor impairments mentioned above.
Interactions
Valnoctamide is known to increase through inhibition of epoxide hydrolase the serum levels of carbamazepine-10,11-epoxide, the active metabolite of carbamazepine, sometimes to toxic levels.[9]
Chemistry
Valnoctamide is a racemic compound with four stereoisomers, all of which were shown to be more effective than valproic acid in animal models of epilepsy and one of which ((2S,3S)-valnoctamide) was considered to be a good candidate by Isoherranen, et al for an anticonvulsant in August of 2003.[10]
Notes and references
- ↑ Haj-Yehia, Abdullah (1988). "Pharmacokinetics of a valpromide isomer, valnoctamide, in dogs". Journal of Pharmaceutical Science. 77 (10): 831–4. PMID 3148708. Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - ↑ Template:Fr icon HARL, F. M. (1964). "[CLINICAL STUDY OF VALNOCTAMIDE ON 70 NEUROPSYCHIATRIC CLINIC PATIENTS UNDERGOING AMBULATORY TREATMENT.]". La Presse Médicale. 72: 753-4. PMID 14119722. Unknown parameter
|month=ignored (help) - ↑ Haj-Yehia, Abdullah (1989). "Structure-pharmacokinetic relationships in a series of valpromide derivatives with antiepileptic activity". Pharmaceutical Research. 6 (8): 683–9. PMID 2510141. Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - ↑ Template:Pt icon Mattos Nda, S. (1969). "[Use of Valnoctamide (nirvanil) in oligophrenic erethics and epileptics.]". Hospital (Rio J). 75 (5): 1701–4. PMID 5306499. Unknown parameter
|month=ignored (help) - ↑ Lindekens (2000). "In vivo study of the effect of valpromide and valnoctamide in the pilocarpine rat model of focal epilepsy". Pharmaceutical Research. 17 (11): 1408–13. PMID 11205735. Text " Hilde " ignored (help); Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - ↑ Winkler, Ilan (2005). "Efficacy of antiepileptic isomers of valproic acid and valpromide in a rat model of neuropathic pain". British Journal of Pharmacology. PMID 15997234. Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help) - ↑ RH Belmaker, Yuly Bersudsky, Alex Mishory and Beersheva Mental Health Center (2005). "Valnoctamide in Mania". ClinicalTrials.gov. United States National Institutes of Health. Unknown parameter
|accessyear=ignored (|access-date=suggested) (help); Unknown parameter|accessdaymonth=ignored (help) - ↑ VALNOCTAMIDE Biam French.
- ↑ Pisani F, Fazio A, Artesi C, Oteri G, Spina E, Tomson T, Perucca E. "Impairment of carbamazepine-10, 11-epoxide elimination by valnoctamide, a valpromide isomer, in healthy subjects." British Journal of Clinical Pharmacology. 1992 Jul;34(1):85-7. PMID 1352988
- ↑ Isoherranen, Nina (2003). "Pharmacokinetic-pharmacodynamic relationships of (2S,3S)-valnoctamide and its stereoisomer (2R,3S)-valnoctamide in rodent models of epilepsy". Pharmaceutical Research. 8 (8): 1293–301. PMID 12948028. Unknown parameter
|coauthors=ignored (help); Unknown parameter|month=ignored (help)
External links
- Pages with script errors
- Pages with citations using unsupported parameters
- Pages with citations using unnamed parameters
- CS1 maint: Multiple names: authors list
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Amides
- Anticonvulsants
- Anxiolytics
- Drug