Valganciclovir hydrochloride clinical pharmacology
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]
Clinical Pharmacology
Pharmacokinetics
Because the major elimination pathway for ganciclovir is renal, dosage reductions according to creatinine clearance are required for Valcyte tablets and Valcyte for oral solution [see Dosage and Administration (2.5)].
Pharmacokinetics in Adults: The pharmacokinetics of valganciclovir and ganciclovir after administration of valganciclovir tablets have been evaluated in HIV- and CMV-seropositive patients, patients with AIDS and CMV retinitis, and in solid organ transplant patients.
The ganciclovir pharmacokinetic parameters following administration of 900 mg Valcyte tablets and 5 mg/kg intravenous ganciclovir and 1000 mg three times daily oral ganciclovir in HIV-positive/CMV-positive patients are summarized in Table 9.
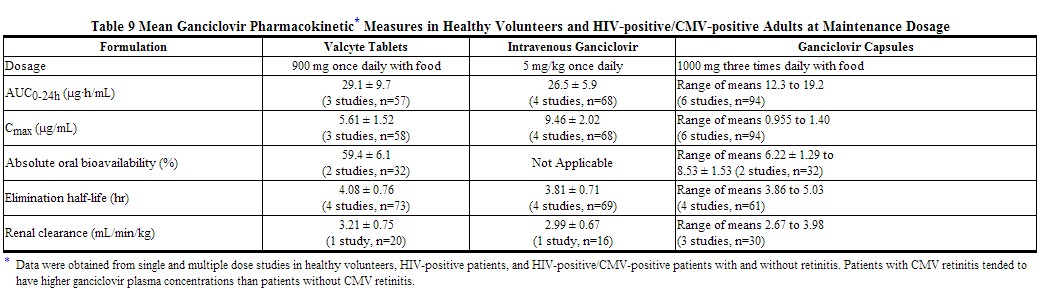 |
The area under the plasma concentration-time curve (AUC) of ganciclovir administered as Valcyte tablets (900 mg once daily) is comparable to the AUC of ganciclovir after administration of intravenous ganciclovir (5 mg/kg once daily). The Cmax of ganciclovir following Valcyte administration is 40% lower than the Cmax following intravenous ganciclovir administration. During maintenance dosing, ganciclovir AUC0-24h and Cmax following oral ganciclovir administration (1000 mg three times daily) are lower relative to Valcyte and intravenous ganciclovir. The ganciclovir Cmin following intravenous ganciclovir and Valcyte administration are less than the ganciclovir Cminfollowing oral ganciclovir administration. The clinical significance of the differences in ganciclovir pharmacokinetics after administration of Valcyte tablets, ganciclovir capsules, and intravenous ganciclovir is unknown.
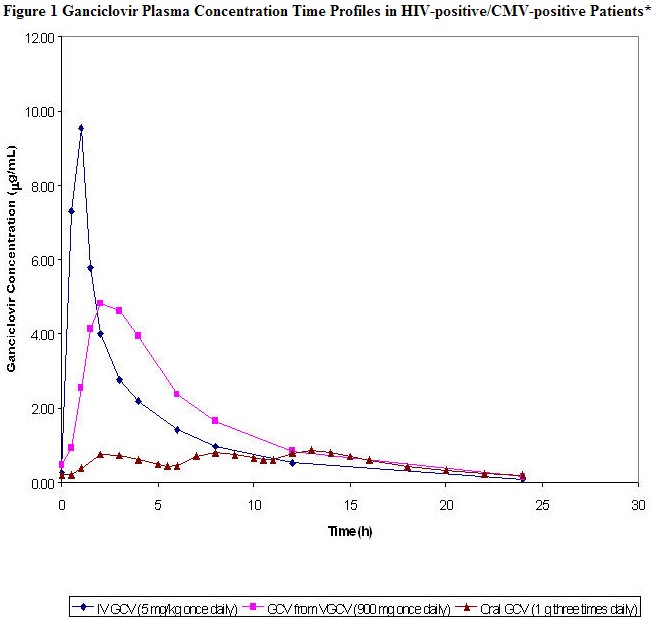 |
- Plasma concentration-time profiles for ganciclovir (GCV) from valganciclovir (VGCV) and intravenous ganciclovir were obtained from a multiple dose study (n=21 and n=18, respectively) in HIV-positive/CMV-positive patients with CMV retinitis. The plasma concentration-time profile for oral ganciclovir was obtained from a multiple dose study (n=24) in HIV-positive/CMV-positive patients without CMV retinitis.
In solid organ transplant recipients, the mean systemic exposure to ganciclovir was 1.7x higher following administration of 900 mg Valcyte tablets once daily versus 1000 mg ganciclovir capsules three times daily, when both drugs were administered according to their renal function dosing algorithms. The systemic ganciclovir exposures attained were comparable across kidney, heart and liver transplant recipients based on a population pharmacokinetics evaluation (see Table 10).
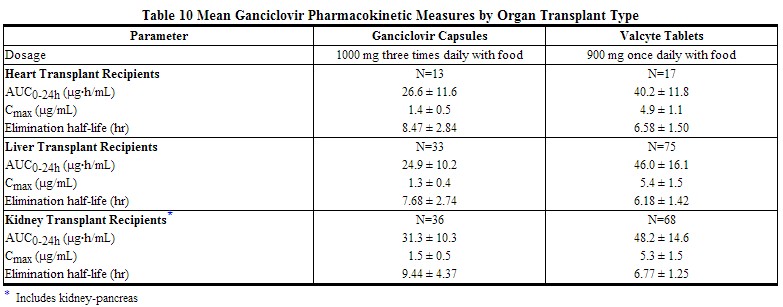 |
The pharmacokinetic parameters of ganciclovir following 200 days of Valcyte administration in high-risk kidney transplant patients were similar to those previously reported in solid organ transplant patients who received Valcyte for 100 days.
In a pharmacokinetic study in liver transplant patients, the ganciclovir AUC0-24h achieved with 900 mg valganciclovir was 41.7 ± 9.9 µg∙h/mL (n=28) and the AUC0-24hachieved with the approved dosage of 5 mg/kg intravenous ganciclovir was 48.2 ± 17.3 µg∙h/mL (n=27).
Absorption: Valganciclovir, a prodrug of ganciclovir, is well absorbed from the gastrointestinal tract and rapidly metabolized in the intestinal wall and liver to ganciclovir. The absolute bioavailability of ganciclovir from Valcyte tablets following administration with food was approximately 60% (3 studies, n=18; n=16; n=28). Ganciclovir median Tmax following administration of 450 mg to 2625 mg Valcyte tablets ranged from 1 to 3 hours. Dose proportionality with respect to ganciclovir AUC following administration of Valcyte tablets was demonstrated only under fed conditions. Systemic exposure to the prodrug, valganciclovir, is transient and low, and the AUC24 and Cmax values are approximately 1% and 3% of those of ganciclovir, respectively.
Food Effects: When Valcyte tablets were administered with a high fat meal containing approximately 600 total calories (31.1 g fat, 51.6 g carbohydrates and 22.2 g protein) at a dose of 875 mg once daily to 16 HIV-positive subjects, the steady-state ganciclovir AUC increased by 30% (95% CI 12% to 51%), and the Cmax increased by 14% (95% CI -5% to 36%), without any prolongation in time to peak plasma concentrations (Tmax). Valcyte should be administered with food [see Dosage and Administration (2.1)].
Distribution: Due to the rapid conversion of valganciclovir to ganciclovir, plasma protein binding of valganciclovir was not determined. Plasma protein binding of ganciclovir is 1% to 2% over concentrations of 0.5 and 51 µg/mL. When ganciclovir was administered intravenously, the steady-state volume of distribution of ganciclovir was 0.703 ± 0.134 L/kg (n=69).
After administration of Valcyte tablets, no correlation was observed between ganciclovir AUC and reciprocal weight; oral dosing of Valcyte tablets according to weight is not required.
Metabolism: Valganciclovir is rapidly hydrolyzed to ganciclovir; no other metabolites have been detected. No metabolite of orally administered radiolabeled ganciclovir (1000 mg single dose) accounted for more than 1% to 2% of the radioactivity recovered in the feces or urine.
Elimination: The major route of elimination of valganciclovir is by renal excretion as ganciclovir through glomerular filtration and active tubular secretion. Systemic clearance of intravenously administered ganciclovir was 3.07 ± 0.64 mL/min/kg (n=68) while renal clearance was 2.99 ± 0.67 mL/min/kg (n=16).
The terminal half-life (t½) of ganciclovir following oral administration of Valcyte tablets to either healthy or HIV-positive/CMV-positive subjects was 4.08 ± 0.76 hours (n=73), and that following administration of intravenous ganciclovir was 3.81 ± 0.71 hours (n=69). In heart, kidney, kidney-pancreas, and liver transplant patients, the terminal elimination half-life of ganciclovir following oral administration of Valcyte was 6.48 ± 1.38 hours, and following oral administration of ganciclovir capsules was 8.56 ± 3.62 hours.
Specific Populations
Renal Impairment: The pharmacokinetics of ganciclovir from a single oral dose of 900 mg Valcyte tablets were evaluated in 24 otherwise healthy individuals with renal impairment.
| thumb|800px|left |
Decreased renal function results in decreased clearance of ganciclovir from valganciclovir, and a corresponding increase in terminal half-life. Therefore, dosage adjustment is required for patients with impaired renal function.
Hemodialysis reduces plasma concentrations of ganciclovir by about 50% following Valcyte administration. Adult patients receiving hemodialysis (CrCl <10 mL/min) cannot use Valcyte tablets because the daily dose of Valcyte tablets required for these patients is less than 450 mg [see Dosage and Administration (2.5) and Use in Specific Populations (8.6)].
Pharmacokinetics in Pediatric Patients: The pharmacokinetics of ganciclovir were evaluated following the administration of valganciclovir in 63 pediatric solid organ transplant patients aged 4 months to 16 years. In this study, patients received oral doses of valganciclovir (either Valcyte for oral solution or tablets) to produce exposure equivalent to an adult 900 mg dose [see Dosage and Administration (2.3), Adverse Reactions (6.2), Use in Specific Populations (8.4), Clinical Studies (14.2)].
The pharmacokinetics of ganciclovir were similar across organ types and age ranges. Population pharmacokinetic modeling suggested that bioavailability was approximately 60%. Clearance was positively influenced by both body surface area and renal function. The mean total clearance was 5.3 L/hr (88.3 mL/min) for a patient with creatinine clearance of 70.4 mL/min. The mean Cmax and AUC by age and organ type are listed in Table 12.
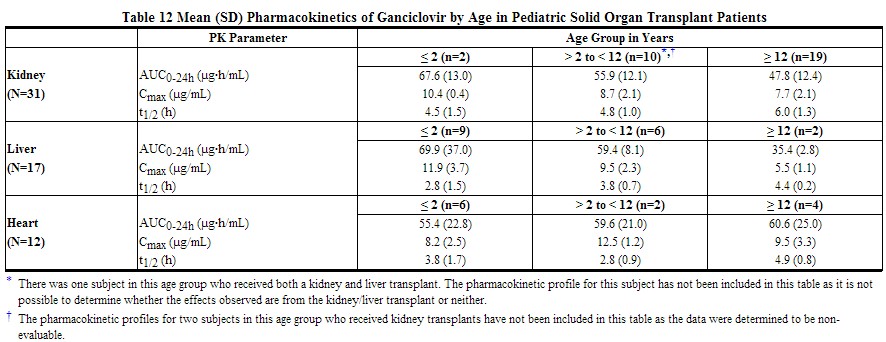 |
Pharmacokinetics in Geriatric Patients: The pharmacokinetic characteristics of Valcyte in elderly patients have not been established. Because elderly individuals frequently have a reduced glomerular filtration rate, renal function should be assessed before and during administration of Valcyte [see Dosage and Administration (2.5),Use in Specific Populations (8.5)].
Drug Interactions
In vivo drug-drug interaction studies were not conducted with valganciclovir. However, because valganciclovir is rapidly and extensively converted to ganciclovir, interactions associated with ganciclovir will be expected for Valcyte [see Drug Interactions (7)].
Drug-drug interaction studies were conducted in patients with normal renal function. Patients with impaired renal function may have increased concentrations of ganciclovir and the coadministered drug following concomitant administration of Valcyte and drugs excreted by the same pathway as ganciclovir. Therefore, these patients should be closely monitored for toxicity of ganciclovir and the coadministered drug.
Table 13 and Table 14 provide a listing of established drug interaction studies with ganciclovir. Table 13 provides the effects of coadministered drug on ganciclovir plasma pharmacokinetic parameters, whereas Table 14 provides the effects of ganciclovir on plasma pharmacokinetic parameters of coadministered drug.[1]
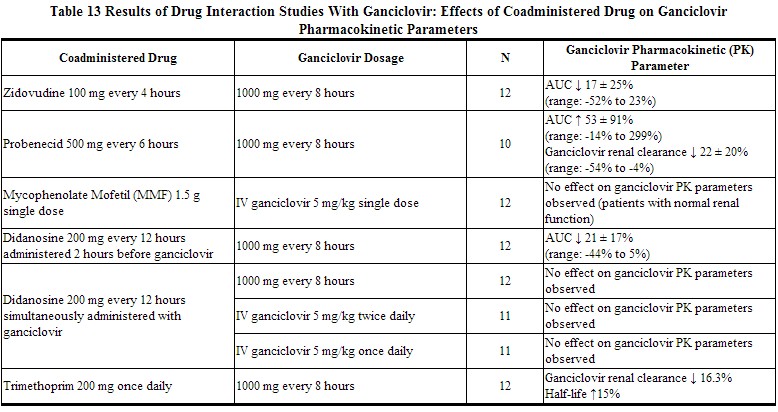 |
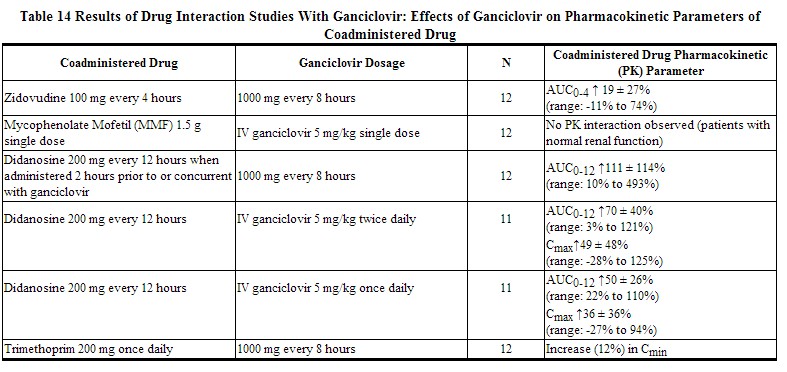 |
References
- ↑ "http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021304s008,022257s003lbl.pdf" (PDF). External link in
|title=(help)
Adapted from the FDA Package Insert.