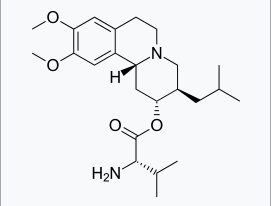
Valbenazine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sonya Gelfand
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Overview
Valbenazine is a Acetylcholine release inhibitor, Adrenergic receptor agonist that is FDA approved for the (type of indication of drug) of a list of indications, separated by commas.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include a list of adverse reactions, separated by commas..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition 1
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Condition 2
- Developed by: (Organisation)
- Class of Recommendation: (Class) (Link)
- Strength of Evidence: (Category A/B/C) (Link)
- Dosing Information/Recommendation
- (Dosage)
Non–Guideline-Supported Use
Condition 1
- Dosing Information
- (Dosage)
Condition 2
- Dosing Information
- (Dosage)
Condition 3
- Dosing Information
- (Dosage)
Contraindications
CONTRAINDICATIONS
Warnings
|
Warning Title
See full prescribing information for complete Boxed Warning.
Condition Name: (Content)
|
Conidition 1
(Description)
Conidition 2
(Description)
Conidition 3
(Description)
Adverse Reactions
Clinical Trials Experience
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Condition 2
Central Nervous System
- (list/description of adverse reactions)
Cardiovascular
- (list/description of adverse reactions)
Respiratory
- (list/description of adverse reactions)
Gastrointestinal
- (list/description of adverse reactions)
Hypersensitive Reactions
- (list/description of adverse reactions)
Miscellaneous
- (list/description of adverse reactions)
Postmarketing Experience
(Description)
Drug Interactions
- Drug 1
- Drug 2
- Drug 3
- Drug 4
- Drug 5
Drug 1
(Description)
Drug 2
(Description)
Drug 3
(Description)
Drug 4
(Description)
Drug 5
(Description)
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
(Description)
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Valbenazine in women who are pregnant.
Labor and Delivery
(Description)
Nursing Mothers
(Description)g
Pediatric Use
(Description)
Geriatic Use
(Description)
Gender
(Description)
Race
(Description)
Renal Impairment
(Description)
Hepatic Impairment
(Description)
Females of Reproductive Potential and Males
(Description)
Immunocompromised Patients
(Description)
Others
(Description)
Administration and Monitoring
Administration
(Oral/Intravenous/etc)
Monitoring
Condition 1
(Description regarding monitoring, from Warnings section)
Condition 2
(Description regarding monitoring, from Warnings section)
Condition 3
(Description regarding monitoring, from Warnings section)
IV Compatibility
There is limited information regarding the compatibility of Valbenazine and IV administrations.
Overdosage
Acute Overdose
Signs and Symptoms
(Description)
Management
(Description)
Chronic Overdose
Signs and Symptoms
(Description)
Management
(Description)
Pharmacology
Mechanism of Action
- The mechanism of action of valbenazine in the treatment of tardive dyskinesia is unknown, but is thought to be mediated through the reversible inhibition of vesicular monoamine transporter 2 (VMAT2), a transporter that regulates monoamine uptake from the cytoplasm to the synaptic vesicle for storage and release.
Structure

Pharmacodynamics
- Valbenazine inhibits human VMAT2 (Ki ~ 150 nM) with no appreciable binding affinity for VMAT1 (Ki > 10 µM). Valbenazine is converted to the active metabolite [+]-α-dihydrotetrabenazine ([+]-α-HTBZ).
- [+]-α-HTBZ also binds with relatively high affinity to human VMAT2 (Ki ~ 3 nM). Valbenazine and [+]-α-HTBZ have no appreciable binding affinity (Ki > 5000 nM) for dopaminergic (including D2), serotonergic (including 5HT2B), adrenergic, histaminergic or muscarinic receptors.
Cardiac Electrophysiology
- INGREZZA may cause an increase in the corrected QT interval in patients who are CYP2D6 poor metabolizers or who are taking a strong CYP2D6 or CYP3A4 inhibitor. An exposure-response analysis of clinical data from two healthy volunteer studies revealed increased QTc interval with higher plasma concentrations of the active metabolite. Based on this model, patients taking an INGREZZA 80 mg dose with increased exposure to the metabolite (e.g., being a CYP2D6 poor metabolizer) may have a mean QT prolongation of 11.7 msec (14.7 msec upper bound of double-sided 90% CI) as compared to otherwise healthy volunteers given INGREZZA, who had a mean QT prolongation of 6.7 msec (8.4 msec).
Pharmacokinetics
- Valbenazine and its active metabolite ([+]-α-HTBZ) demonstrate approximate proportional increases for the area under the plasma concentration versus time curve (AUC) and maximum plasma concentration (Cmax) after single oral doses from 40 mg to 300 mg (i.e., 50% to 375% of the recommended treatment dose).
Absorption
- Following oral administration, the time to reach maximum valbenazine plasma concentration (tmax) ranges from 0.5 to 1.0 hours. Valbenazine reaches steady state plasma concentrations within 1 week. The absolute oral bioavailability of valbenazine is approximately 49%. [+]-α-HTBZ gradually forms and reaches Cmax 4 to 8 hours after administration of INGREZZA.
- Ingestion of a high-fat meal decreases valbenazine Cmax by approximately 47% and AUC by approximately 13%. [+]-α-HTBZ Cmax and AUC are unaffected.
Distribution
- The plasma protein binding of valbenazine and [+]-α-HTBZ are greater than 99% and approximately 64%, respectively. The mean steady state volume of distribution of valbenazine is 92 L.
- Nonclinical data in Long-Evans rats show that valbenazine can bind to melanin-containing structures of the eye such as the uveal tract. The relevance of this observation to clinical use of INGREZZA is unknown.
Elimination
- Valbenazine has a mean total plasma systemic clearance value of 7.2 L/hr. Valbenazine and [+]-α-HTBZ have half-lives of 15 to 22 hours.
Metabolism
- Valbenazine is extensively metabolized after oral administration by hydrolysis of the valine ester to form the active metabolite ([+]-α-HTBZ) and by oxidative metabolism, primarily by CYP3A4/5, to form mono-oxidized valbenazine and other minor metabolites. [+]-α-HTBZ appears to be further metabolized in part by CYP2D6.
- The results of in vitro studies suggest that valbenazine and [+]-α-HTBZ are unlikely to inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2E1 or CYP3A4/5, or induce CYP1A2, CYP2B6 or CYP3A4/5 at clinically relevant concentrations.
- The results of in vitro studies suggest that valbenazine and [+]-α-HTBZ are unlikely to inhibit the transporters (BCRP, OAT1, OAT3, OCT2, OATP1B1, or OATP1B3) at clinically relevant concentrations.
Excretion
- Following the administration of a single 50-mg oral dose of radiolabeled C-valbenazine (i.e., ~63% of the recommended treatment dose), approximately 60% and 30% of the administered radioactivity was recovered in the urine and feces, respectively. Less than 2% was excreted as unchanged valbenazine or [+]-α-HTBZ in either urine or feces.
Studies in Specific Populations
- Exposures of valbenazine in patients with hepatic impairment are summarized in Figure 1.

Drug Interaction Studies
- The effects of ketoconazole and rifampin on the exposure of valbenazine are summarized in Figure 2.

- The effects of valbenazine on the exposure of other coadministered drugs are summarized in Figure 3.

Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
- Valbenazine did not increase tumors in rats treated orally for 91 weeks at 0.5, 1, and 2 mg/kg/day. These doses are <1 times (0.06, 0.1, and 0.24 times, respectively) the MRHD of 80 mg/day based on mg/m2.
- Valbenazine did not increase tumors in hemizygous Tg.rasH2 mice treated orally for 26 weeks at 10, 30 and 75 mg/kg/day, which are 0.6, 1.9 and 4.6 times the MRHD of 80 mg/day based on mg/m2.
Mutagenesis
- Valbenazine was not mutagenic in the in vitro bacterial reverse mutation test (Ames) or clastogenic in the in vitro mammalian chromosomal aberrations assay in human peripheral blood lymphocytes or in the in vivo rat bone marrow micronucleus assay.
Impairment of Fertility
- In a fertility study, rats were treated orally with valbenazine at 1, 3, and 10 mg/kg/day prior to mating and through mating, for a minimum of 10 weeks (males) or through Day 7 of gestation (females). These doses are 0.1, 0.4, and 1.2 times the MRHD of 80 mg/day based on mg/m2, respectively. Valbenazine delayed mating in both sexes, which led to lower number of pregnancies and disrupted estrous cyclicity at the high dose, 1.2 times the MRHD of 80 mg/day based on mg/m2. Valbenazine had no effects on sperm parameters (motility, count, density) or on uterine parameters (corpora lutea, number of implants, viable implants, pre-implantation loss, early resorptions and post-implantation loss) at any dose.
Clinical Studies
- A randomized, double-blind, placebo-controlled trial of INGREZZA was conducted in patients with moderate to severe tardive dyskinesia as determined by clinical observation. Patients had underlying schizophrenia, schizoaffective disorder, or a mood disorder. Individuals at significant risk for suicidal or violent behavior and individuals with unstable psychiatric symptoms were excluded.
- The Abnormal Involuntary Movement Scale (AIMS) was the primary efficacy measure for the assessment of tardive dyskinesia severity. The AIMS is a 12-item scale; items 1 to 7 assess the severity of involuntary movements across body regions and these items were used in this study. Each of the 7 items was scored on a 0 to 4 scale, rated as: 0=no dyskinesia; 1=low amplitude, present during some but not most of the exam; 2=low amplitude and present during most of the exam (or moderate amplitude and present during some of the exam); 3=moderate amplitude and present during most of exam; or 4=maximal amplitude and present during most of exam. The AIMS dyskinesia total score (sum of items 1 to 7) could thus range from 0 to 28, with a decrease in score indicating improvement. The AIMS was scored by central raters who interpreted the videos blinded to subject identification, treatment assignment, and visit number.
- The primary efficacy endpoint was the mean change from baseline in the AIMS dyskinesia total score at the end of Week 6. The change from baseline for two fixed doses of INGREZZA (40 mg or 80 mg) was compared to placebo. At the end of Week 6, subjects initially assigned to placebo were re-randomized to receive INGREZZA 40 mg or 80 mg. Subjects originally randomized to INGREZZA continued INGREZZA at their randomized dose. Follow-up was continued through Week 48 on the assigned drug, followed by a 4-week period off-drug (subjects were not blind to withdrawal).
- A total of 234 subjects were enrolled, with 29 (12%) discontinuing prior to completion of the placebo-controlled period. Mean age was 56 (range 26 to 84). Patients were 54% male and 46% female. Patients were 57% Caucasian, 38% African-American, and 5% other. Concurrent diagnoses included schizophrenia/schizoaffective disorder (66%) and mood disorder (34%). With respect to concurrent antipsychotic use, 70% of subjects were receiving atypical antipsychotics, 14% were receiving typical or combination antipsychotics, and 16% were not receiving antipsychotics.
- Results are presented in Table 3, with the distribution of responses shown in Figure 4. The change from baseline in the AIMS total dyskinesia score in the 80 mg INGREZZA group was statistically significantly different from the change in the placebo group. Subgroup analyses by gender, age, racial subgroup, underlying psychiatric diagnostic category, and concomitant antipsychotic medication did not suggest any clear evidence of differential responsiveness.
- The mean changes in the AIMS dyskinesia total score by visit are shown in Figure 5. Among subjects remaining in the study at the end of the 48-week treatment (N=123 [52.6%]), following discontinuation of INGREZZA, the mean AIMS dyskinesia total score appeared to return toward baseline (there was no formal hypothesis testing for the change following discontinuation).



How Supplied
- INGREZZA (valbenazine) capsules are available as:
- 40 mg Capsule: White opaque body with a purple cap, printed with ‘VBZ’ and ‘40’ in black ink.
- Bottle of 30: NDC 70370-1040-1
- Bottle of 90: NDC 70370-1040-2
- 80 mg Capsule: Purple opaque body and cap, printed with ‘VBZ’ and ‘80’ in black ink.
- Bottle of 30: NDC 70370-1080-1
Storage
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F).
Images
Drug Images
{{#ask: Page Name::Valbenazine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Valbenazine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling.
Somnolence
- Inform patients that INGREZZA may cause somnolence and may impair the ability to perform tasks that require complex motor and mental skills. Advise patients that until they learn how they respond to INGREZZA, they should be careful or avoid doing activities that require them to be alert, such as driving a car or operating machinery.
Prolongation of the QT Interval
- Inform patients to consult their physician immediately if they feel faint, lose consciousness, or have heart palpitations. Advise patients to inform physicians that they are taking INGREZZA before any new drug is taken.
Pregnancy
- Advise a pregnant patient of the potential risk to a fetus.
Lactation
- Advise a woman not to breastfeed during treatment with INGREZZA and for 5 days after the final dose.

Precautions with Alcohol
Alcohol-Valbenazine interaction has not been established. Talk to your doctor regarding the effects of taking alcohol with this medication.
Brand Names
- Ingrezza
Look-Alike Drug Names
There is limited information regarding Valbenazine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Drug Shortage
Price
References
The contents of this FDA label are provided by the National Library of Medicine.