Trovafloxacin mesylate: Difference between revisions
Gloria Picoy (talk | contribs) (Created page with "{{DrugProjectFormSinglePage |authorTag={{GP}} |genericName=Trovafloxacin mesylate |blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> |blackBoxWarningBody=<...") |
Gloria Picoy (talk | contribs) No edit summary |
||
| Line 2: | Line 2: | ||
|authorTag={{GP}} | |authorTag={{GP}} | ||
|genericName=Trovafloxacin mesylate | |genericName=Trovafloxacin mesylate | ||
|blackBoxWarningTitle= | |aOrAn=a | ||
|blackBoxWarningBody= | |drugClass=fluoroquinolone | ||
|blackBoxWarningTitle=Warning: Liver injury | |||
|blackBoxWarningBody=Trovafloxacin mesylate has been associated with serious liver injury leading to liver transplantation and/or death. Trovafloxacin mesylate-associated liver injury have been reported with both short-term and long.term drug exposure. Trovafloxacin use exceeding 2 weeks in duration is associated with a significantly increased risk of serious liver injury. Liver injury has also been reported following trovafloxacin re-exposure. Trovafloxacin should be reserved for use in patients with serious, life or limb-threatening infections who receive their initial therapy in an in-patient health care facility (I.E., Hospotal or long-term nursing care facility). Trovafloxacin should not be used when safer, alternative antimicrobial therapy will be effective. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Trovafloxacin mesylate in adult patients. | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Trovafloxacin mesylate in adult patients. | ||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Trovafloxacin mesylate in adult patients. | |offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Trovafloxacin mesylate in adult patients. | ||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Trovafloxacin mesylate in pediatric patients. | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Trovafloxacin mesylate in pediatric patients. | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Trovafloxacin mesylate in pediatric patients. | |offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Trovafloxacin mesylate in pediatric patients. | ||
|contraindications=Trovafloxacin mesylate is contraindicated in persons with a history of hypersensitivity to trovafloxacin, alatrofloxacin, quinolone antimicrobial agents or any other components of these products. | |||
|warnings=Trovafloxacin mesylate-associated liver enzyme abnormalities, symptomatic hepatitis, jaundice and liver failure (including rare reports of acute hepatic necrosis with eosinophilic infiltration, liver transplantation and/or death) have been reported with both short-term and long-term drug exposure in men and women. Trovafloxacin use exceeding 2 weeks in duration is associated with a significantly increased risk of serious liver injury. Liver injury has also benn reportes following trovafloxacin re-exposure. clinicians should monitor liver function test (e.g., AST, ALT, bilirubin) in trovafloxacin recipients who develop signs or symptoms consistent with hepatitis. Clinicians should consider discontinuing travofloxacin in those patients who develop liver function test abnormalities. | |||
The safety and effectiveness of trovafloxacin in pediatric patients and adolescents less than 18 years age, pregnant women, and nursing women have not been established. | |||
As with other members of the quinolone class, trovafloxacin has caused arthropathy and/or chondrodysplasia in immature rats and dogs. The significance of these findings to humans is unknown. (See ANIMAL PHARMACOLOGY.) | |||
Convulsions, increased intracranial pressure and psychosis have been reported in patients receiving quinolones. Quinolones may also cause central nervous system stimulation which may lead to tremors, restlessness, lightheadedness, confusion, hallucinations, paranoia, depression, nightmares and insomnia. These reactions may occur following the first dose. If these reactions occur in patients receiving trovafloxacin or alatrofloxacin, the drug should be discontinued and appropriate measures instituted. (See PRECAUTIONS: GENERAL,INFORMATION FOR PATIENTS, DRUG INTERACTIONS and ADVERSE REACTIONS.) | |||
As with other quinolones, TROVAN should be used with caution in patients with known or suspected CNS disorders, such as severe cerebral atherosclerosis, epilepsy, and other factors that predispose to seizures. (See ADVERSE REACTIONS.) | |||
Serious and occasionally fatal hypersensitivity and/or anaphylactic reactions have been reported in patients receiving therapy with TROVAN. These reactions may occur following the first dose. Some reactions have been accompanied by cardiovascular collapse, hypotension/shock, seizure, loss of consciousness, tingling, angioedema (including tongue, laryngeal, throat or facial edema/swelling), airway obstruction (including bronchospasm, shortness of breath and acute respiratory distress), dyspnea, urticaria, itching and other serious skin reactions, including generalized erythema. | |||
Life-threatening hypotension has been reported with alatrofloxacin administration. This has occurred in patients receiving alatrofloxacin at either the recommended rate of infusion or if given more rapidly. Hypotension may be potentiated with the concomitant administration of anesthetic agents. Alatrofloxacin should only be administered by slow intravenous infusion over a period of 60 minutes. Blood pressure should be monitored closely during infusion. | |||
TROVAN should be discontinued at the first appearance of a skin rash or any other sign of hypersensitivity. Serious acute hypersensitivity reactions may require treatment with epinephrine and other resuscitative measures, including oxygen, intravenous fluids, antihistamines, corticosteroids, pressor amines and airway management, as clinically indicated. (See PRECAUTIONS and ADVERSE REACTIONS.) | |||
Serious and sometimes fatal events, some due to hypersensitivity and some due to uncertain etiology, have been reported in patients receiving therapy with all antibiotics. These events may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following: fever, rash or severe dermatologic reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson Syndrome); vasculitis, arthralgia, myalgia, serum sickness; allergic pneumonitis, interstitial nephritis; acute renal insufficiency or failure; hepatitis, jaundice, acute hepatic necrosis or failure; anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities. | |||
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including TROVAN, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of any antibacterial agent. | |||
Treatment with antibacterial agents alters the flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is the primary cause of "antibiotic-associated colitis." | |||
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against C. difficile colitis. (See ADVERSE REACTIONS.) | |||
Although not seen in TROVAN clinical trials, ruptures of the shoulder, hand, and Achilles tendons that required surgical repair or resulted in prolonged disability have been reported in patients receiving quinolones. TROVAN should be discontinued if the patient experiences pain, inflammation or rupture of a tendon. Patients should rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture has been confidently excluded. Tendon rupture can occur during or after therapy with quinolones. | |||
Trovafloxacin has not been shown to be effective in the treatment of syphilis. Antimicrobial agents used in high doses for short periods of time to treat gonorrhea may mask or delay the symptoms of incubating syphilis. All patients with gonorrhea should have a serologic test for syphilis at the time of diagnosis. | |||
|clinicalTrials=Over 6000 patients have been treated with TROVAN in multidose clinical efficacy trials worldwide. | |||
In TROVAN studies the majority of adverse reactions were described as mild in nature (over 90% were described as mild or moderate). TROVAN was discontinued for adverse events thought related to drug in 5% of patients (dizziness 2.4%, nausea 1.9%, headache 1.1%, and vomiting 1.0%). | |||
[[File:Trovafloxacin mesylate Adverse reactions.png|thumb|none|600px]] | |||
Dizziness/lightheadedness on TROVAN is generally mild, lasts for a few hours following a dose, and in most cases, resolves with continued dosing. The incidence of dizziness and lightheadedness in TROVAN patients over 65 years is 3.1% and 0.6%, respectively. | |||
TROVAN appears to have a low potential for phototoxicity. In clinical trials with TROVAN, only mild, treatment-related phototoxicity was observed in less than 0.03% (2/7096) of patients. | |||
Additional reported drug-related events in clinical trials (remotely, possibly, probably or unknown) that occurred in <1% of TROVAN-treated patients are: | |||
APPLICATION/INJECTION/INSERTION SITE: Application/injection/insertion site device complications, inflammation, pain, edema | |||
AUTONOMIC NERVOUS: flushing, increased sweating, dry mouth, cold clammy skin, increased saliva | |||
CARDIOVASCULAR: peripheral edema, chest pain, thrombophlebitis, hypotension, palpitation, periorbital edema, hypertension, syncope, tachycardia, angina pectoris, bradycardia, peripheral ischemia, edema, dizziness postural | |||
CENTRAL & PERIPHERAL NERVOUS SYSTEM: confusion, paresthesia, vertigo, hypoesthesia, ataxia, convulsions, dysphonia, hypertonia, migraine, involuntary muscle contractions, speech disorder, encephalopathy, abnormal gait, hyperkinesia, hypokinesia, tongue paralysis, abnormal coordination, tremor, dyskinesia | |||
GASTROINTESTINAL: altered bowel habit, constipation, diarrhea-Clostridium difficile, dyspepsia, flatulence, loose stools, gastritis, dysphagia, increased appetite, gastroenteritis, rectal disorder, colitis, pseudomembranous colitis, enteritis, eructation, gastrointestinal disorder, melena, hiccup | |||
ORAL CAVITY: gingivitis, stomatitis, altered saliva, tongue disorder, tongue edema, tooth disorder, cheilitis, halitosis | |||
GENERAL/OTHER: fever, fatigue, pain, asthenia, moniliasis, hot flushes, back pain, chills, infection (bacterial, fungal), malaise, sepsis, alcohol intolerance, allergic reaction, anaphylactoid reaction, drug (other) toxicity/reaction, weight increase, weight decrease | |||
HEMATOPOIETIC: anemia, granulocytopenia, hemorrhage unspecified, leukopenia, prothrombin decreased, thrombocythemia, thrombocytopenia | |||
LIVER/BILIARY: increased hepatic enzymes, hepatic function abnormal, bilirubinemia, discolored feces, jaundice | |||
METABOLIC/NUTRITIONAL: hyperglycemia, thirst | |||
MUSCULOSKELETAL: arthralgia, muscle cramps, myalgia, muscle weakness, skeletal pain, tendinitis, arthropathy | |||
PSYCHIATRIC: anxiety, anorexia, agitation, nervousness, somnolence, insomnia, depression, amnesia, concentration impaired, depersonalization, dreaming abnormal, emotional lability, euphoria, hallucination, impotence, libido decreased-male, paroniria, thinking abnormal | |||
REPRODUCTIVE: Female: leukorrhea, menstrual disorder; Male: balanoposthitis | |||
RESPIRATORY: dyspnea, rhinitis, sinusitis, bronchospasm, coughing, epistaxis, respiratory insufficiency, upper respiratory tract infection, respiratory disorder, asthma, hemoptysis, hypoxia, stridor | |||
SKIN/APPENDAGES: pruritus ani, skin disorder, skin ulceration, angioedema, dermatitis, dermatitis fungal, photosensitivity skin reaction, seborrhea, skin exfoliation, urticaria | |||
SPECIAL SENSES: taste perversion, eye pain, abnormal vision, conjunctivitis, photophobia, conjuctival hemorrhage, hyperacusis, scotoma, tinnitus, visual field defect, diplopia, xerophthalmia | |||
URINARY SYSTEM: dysuria, face edema, micturition frequency, interstitial nephritis, renal failure acute, renal function abnormal, urinary incontinence | |||
LABORATORY CHANGES: Changes in laboratory parameters, without regard to drug relationship, occurring in ≥1% of TROVAN-treated patients were: decreased hemoglobin and hematocrit; increased platelets; decreased and increased WBC; eosinophilia; increased ALT (SGPT), AST (SGOT), and alkaline phosphatase; decreased protein and albumin; increased BUN and creatinine; decreased sodium; and bicarbonate. It is not known whether these abnormalities were caused by the drug or the underlying condition being treated. | |||
The incidence and magnitude of liver function abnormalities with TROVAN were the same as comparator agents except in the only study in which oral TROVAN was administered for 28 days. In this study (chronic bacterial prostatitis) nine percent (13/140) of TROVAN-treated patients experienced elevations of serum transaminases (AST and/or ALT) of ≥3 times the upper limit of normal. These liver function test abnormalities generally developed at the end of, or following completion of, the planned 28-day course of therapy, but were not associated with concurrent elevations of related laboratory measures of hepatic function (such as serum bilirubin, alkaline phosphatase, or lactate dehydrogenase). Patients were asymptomatic with these abnormalities, which generally returned to normal within 1–2 months after discontinuation of therapy. | |||
|postmarketing=Adverse reactions reported with TROVAN during the post-marketing period include: | |||
* GASTROINTESTINAL: symptomatic pancreatitis. | |||
* GENERAL/OTHER: anaphylaxis, Stevens-Johnson Syndrome. | |||
* HEMATOPOIETIC: agranulocytosis, aplastic anemia, pancytopenia. | |||
* LIVER/BILIARY: symptomatic hepatitis (some patients experienced an associated peripheral eosinophilia), liver failure (including acute hepatic necrosis with eosinophilic infiltration). TROVAN-associated liver enzyme abnormalities and/or symptomatic hepatitis have occurred during short-term or long-term therapy. | |||
|drugInteractions=* Antacids, Sucralfate, and Iron: The absorption of oral trovafloxacin is significantly reduced by the concomitant administration of some antacids containing magnesium or aluminum, citric acid/sodium citrate (Bicitra®), as well as sucralfate and iron (ferrous ions). These agents as well as formulations containing divalent and trivalent cations such as Videx®, (Didanosine), chewable/buffered tablets or the pediatric powder for oral solution, should be taken at least 2 hours before or 2 hours after oral trovafloxacin administration. | |||
* Morphine: Co-administration of intravenous morphine significantly reduces the absorption of oral trovafloxacin. Intravenous morphine should be administered at least 2 hours after oral TROVAN dosing in the fasted state and at least 4 hours after oral TROVAN is taken with food. Trovafloxacin administration had no effect on the pharmacokinetics of morphine or its metabolite, morphine-6-β-glucuronide. | |||
* Warfarin: There have been reports during the post-marketing experience that trovafloxacin/alatrofloxacin enhance the effects of warfarin, including cases of bleeding. The mechanism for this reaction is unknown. Prothrombin time, International Normalized Ratio (INR) or other suitable anticoagulation tests should be closely monitored if trovafloxacin/alatrofloxacin is administered concomitantly with warfarin. Patients should also be monitored for evidence of bleeding. | |||
* Minor pharmacokinetic interactions without clinical significance have been observed with co-administration of TROVAN Tablets with caffeine, omeprazole and calcium carbonate. | |||
* No significant pharmacokinetic interactions with theophylline, cimetidine, digoxin, warfarin, or cyclosporine have been observed with TROVAN Tablets. | |||
* Alatrofloxacin should not be co-administered with any solution containing multivalent cations, e.g., magnesium, through the same intravenous line. | |||
|FDAPregCat=C | |||
|useInPregnancyFDA=An increase in skeletal variations was observed in rat fetuses after daily oral 75 mg/kg maternal doses of trovafloxacin (approximately 15 times the highest recommended human dose based on mg/kg or 2 times based upon body surface area) were administered during organogenesis. However, fetal skeletal variations were not observed in rats dosed orally with 15 mg/kg trovafloxacin. Evidence of fetotoxicity (increased perinatal mortality and decreased body weights) was also observed in rats at 75 mg/kg. Daily oral doses of trovafloxacin at 45 mg/kg (approximately 9 times the highest recommended human dose based on mg/kg or 2.7 times based upon body surface area) in the rabbit were not associated with an increased incidence of fetal skeletal variations or malformations. | |||
An increase in skeletal variations and malformations was observed in rat fetuses after daily intravenous doses of alatrofloxacin at ≥20 mg/kg/day (approximately 4 times the highest recommended human dose based on mg/kg or 0.6 times based upon body surface area) were administered to dams during organogenesis. In the rabbit, an increase in fetal skeletal malformations was also observed when 20 mg/kg/day (approximately equal to the highest recommended human dose based upon body surface area) of alatrofloxacin was given intravenously during the period of organogenesis. Intravenous dosing of alatrofloxacin at 6.5 mg/kg in the rat or rabbit was not associated with an increased incidence of skeletal variations or malformations. Fetotoxicity and fetal skeletal malformations have been associated with other quinolones. | |||
Oral doses of trovafloxacin >5mg/kg were associated with an increased gestation time in rats, and several dams at 75 mg/kg experienced uterine dystocia. | |||
There are no adequate and well-controlled studies in pregnant women. TROVAN should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
|useInNursing=Trovafloxacin is excreted in human milk and was found in measurable concentrations in the breast milk of lactating subjects. | |||
Because of the potential for unknown effects from trovafloxacin in nursing infants from mothers taking trovafloxacin, a decision should be made either to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |||
|useInPed=The safety and effectiveness of trovafloxacin in pediatric patients and adolescents less than 18 years of age have not been established. Quinolones, including trovafloxacin, cause arthropathy and osteochondrosis in juvenile animals of several species. | |||
|useInGeri=In multiple-dose clinical trials of trovafloxacin, 27% of patients were ≥65 years of age and 12% of patients were ≥75 years of age. The overall incidence of drug-related adverse reactions, including central nervous system and gastrointestinal side effects, was less in the ≥65 year group than the other age groups. | |||
|useInGender=There are no significant differences in trovafloxacin pharmacokinetics between males and females when differences in body weight are taken into account. After single 200 mg doses, trovafloxacin Cmax and AUC(0–∞) were 60% and 32% higher, respectively, in healthy females compared to healthy males. Following repeated daily administration of 200 mg for 7 days, the Cmax for trovafloxacin was 38% higher and AUC(0–24) was 16% higher in healthy females compared to healthy males. The clinical importance of the increases in serum levels of trovafloxacin in females has not been established. | |||
|useInRenalImpair=The pharmacokinetics of trovafloxacin are not affected by renal impairment. Trovafloxacin serum concentrations are not significantly altered in subjects with severe renal insufficiency (creatinine clearance <20 mL/min), including patients on hemodialysis. | |||
|useInHepaticImpair=Following repeated administration of 100 mg for 7 days to patients with mild cirrhosis (Child-Pugh Class A), the AUC(0–24) for trovafloxacin was increased ~45% compared to matched controls. Repeated administration of 200 mg for 7 days to patients with moderate cirrhosis (Child-Pugh Class B) resulted in an increase of ~50% in AUC(0–24) compared to matched controls. There appeared to be no significant effect on trovafloxacin Cmax for either group. The oral clearance of trovafloxacin was reduced ~30% in both cirrhosis groups, which corresponded to prolongation of half life by 2–2.5 hours (25–30% increase) compared to controls. There are no data in patients with severe cirrhosis (Child-Pugh Class C). Dosage adjustment is recommended in patients with mild to moderate cirrhosis. | |||
|useInReproPotential=Trovafloxacin and alatrofloxacin did not affect the fertility of male or female rats at oral and I.V. doses of 75 mg/kg/day and 50 mg/kg/day, respectively. These doses are 15 and 10 times the recommended maximum human dose based on mg/kg or approximately 2 times based on mg/m2. However, oral doses of trovafloxacin at 200 mg/kg/day (40 times the recommended maximum human dose based on mg/kg or about 6 times based on mg/m2) were associated with increased preimplantation loss in rats. | |||
|othersTitle=Photosensitivity Potential | |||
|useInOthers=In a study of the skin response to ultraviolet and visible radiation conducted in 48 healthy volunteers (12 per group), the minimum erythematous dose (MED) was measured for ciprofloxacin, lomefloxacin, trovafloxacin and placebo before and after drug administration for 5 days. In this study, trovafloxacin (200 mg q.d.) was shown to have a lower potential for producing delayed photosensitivity skin reactions than ciprofloxacin (500 mg b.i.d.) or lomefloxacin (400 mg q.d.), although greater than placebo. | |||
|administration=Intravenous | |||
|mechAction=Trovafloxacin is a fluoronaphthyridone related to the fluoroquinolones with in vitro activity against a wide range of gram-negative and gram-positive aerobic, and anaerobic microorganisms. The bactericidal action of trovafloxacin results from inhibition of DNA gyrase and topoisomerase IV. DNA gyrase is an essential enzyme that is involved in the replication, transcription and repair of bacterial DNA. Topoisomerase IV is an enzyme known to play a key role in the partitioning of the chromosomal DNA during bacterial cell division. Mechanism of action of fluoroquinolones including trovafloxacin is different from that of penicillins, cephalosporins, aminoglycosides, macrolides, and tetracyclines. Therefore, fluoroquinolones may be active against pathogens that are resistant to these antibiotics. There is no cross-resistance between trovafloxacin and the mentioned classes of antibiotics. The overall results obtained from in vitro synergy studies, testing combinations of trovafloxacin with beta-lactams and aminoglycosides, indicate that synergy is strain specific and not commonly encountered. This agrees with results obtained previously with other fluoroquinolones. Resistance to trovafloxacin in vitro develops slowly via multiple-step mutation in a manner similar to other fluoroquinolones. Resistance to trovafloxacin in vitro occurs at a general frequency of between 1×10-7 to 10-10. Although cross-resistance has been observed between trovafloxacin and some other fluoroquinolones, some microorganisms resistant to other fluoroquinolones may be susceptible to trovafloxacin. | |||
|structure=The chemical structure is: | |||
[[File:Trovafloxacin mesylate chemical structure.png|thumb|none|500px]] | |||
|PK=After intravenous administration, alatrofloxacin is rapidly converted to trovafloxacin. Plasma concentrations of alatrofloxacin are below quantifiable levels within 5 to 10 minutes of completion of a 1 hour infusion. | |||
======Absorption====== | |||
Trovafloxacin is well-absorbed from the gastrointestinal tract after oral administration. The absolute bioavailability is approximately 88%. For comparable dosages, no dosage adjustment is necessary when switching from parenteral to oral administration. | |||
[[File:Trovafloxacin mesylate absorption.png|thumb|none|500px]] | |||
Figure 1. Mean trovafloxacin serum concentrations determined following 1 hour intravenous infusions of alatrofloxacin at daily doses of 200 mg (trovafloxacin equivalents) to healthy male volunteers and following daily oral administration of 200 mg trovafloxacin for 7 days to six male and six female healthy young volunteers. | |||
=====Pharmacokinetics Parameters===== | |||
The mean pharmacokinetic parameters (±SD) of trovafloxacin after single and multiple 100 mg and 200 mg oral doses and 1 hour intravenous infusions of alatrofloxacin in doses of 200 and 300 mg (trovafloxacin equivalents) appear in the chart below. | |||
[[File:Trovafloxacin mesylate pharmacokinetics parameters.png|thumb|none|600px]] | |||
Serum concentrations of trovafloxacin are dose proportional after oral administration of trovafloxacin in the dose range of 30 to 1000 mg or after intravenous administration of alatrofloxacin in the dose range of 30 to 400 mg (trovafloxacin equivalents). Steady state concentrations are achieved by the third daily oral or intravenous dose of trovafloxacin with an accumulation factor of approximately 1.3 times the single dose concentrations. | |||
Oral absorption of trovafloxacin is not altered by concomitant food intake; therefore, it can be administered without regard to food. | |||
The systemic exposure to trovafloxacin (AUC0–∞) administered as crushed tablets via nasogastric tube into the stomach was identical to that of orally administered intact tablets. Administration of concurrent enteral feeding solutions had no effect on the absorption of trovafloxacin given via nasogastric tube into the stomach. When trovafloxacin was administered as crushed tablets into the duodenum via nasogastric tube, the AUC0–∞ and peak serum concentration (Cmax) were reduced by 30% relative to the orally administered intact tablets. Time to peak serum level (Tmax) was also decreased from 1.7 hrs to 1.1 hrs. | |||
=====Distribution===== | |||
The mean plasma protein bound fraction is approximately 76%, and is concentration-independent. Trovafloxacin is widely distributed throughout the body. Rapid distribution of trovafloxacin into tissues results in significantly higher trovafloxacin concentrations in most target tissues than in plasma or serum. | |||
[[File:Trovafloxacin mesylate Pharmacokintics Distribution.png|thumb|none|600px]] | |||
=====Presence in Breast Milk===== | |||
Trovafloxacin was found in measurable concentrations in the breast milk of three lactating subjects. The average measurable breast milk concentration was 0.8 µg/mL (range: 0.3–2.1 µg/mL) after single I.V. alatrofloxacin (300 mg trovafloxacin equivalents) and repeated oral trovafloxacin (200 mg) doses. | |||
=====Metabolism===== | |||
Trovafloxacin is metabolized by conjugation (the role of cytochrome P450 oxidative metabolism of trovafloxacin is minimal). Thirteen percent of the administered dose appears in the urine in the form of the ester glucuronide and 9% appears in the feces as the N-acetyl metabolite (2.5% of the dose is found in the serum as the active N-acetyl metabolite). Other minor metabolites (diacid, sulfamate, hydroxycarboxylic acid) have been identified in both urine and feces in small amounts (<4% of the administered dose). | |||
=====Excretion===== | |||
Approximately 50% of an oral dose is excreted unchanged (43% in the feces and 6% in the urine). | |||
After multiple 200 mg doses, to healthy subjects, mean (±SD) cumulative urinary trovafloxacin concentrations were 12.1±3.4 µg/mL. With these levels of trovafloxacin in urine, crystals of trovafloxacin have not been observed in the urine of human subjects. | |||
|nonClinToxic=======Carcinogenesis and Mutagenesis====== | |||
Long term studies in animals to determine the carcinogenic potential of trovafloxacin or alatrofloxacin have not been conducted. | |||
TROVAN did not shorten the time to development of UV-induced skin tumors in hairless albino (Skh-1) mice; thus, it was not photo co-carcinogenic in this model. These mice received oral trovafloxacin and concurrent irradiation with simulated sunlight 5 days per week for 40 weeks followed by a 12-week treatment-free observation period. The daily dose of UV radiation used in this study was approximately 30% of the minimal dose of UV radiation that would induce erythema in Caucasian humans. The median time to the development of skin tumors in the hairless mice (42–43 weeks) was similar in the vehicle control group and those given 10 or 30 mg/kg of trovafloxacin daily. At a dose level of 30 mg/kg/day, the mice had skin trovafloxacin concentrations of approximately 7 µg/g. Following multiple 200 mg daily doses of trovafloxacin, the amount in human skin is estimated to be about 3 µg/g, based upon plasma concentrations measured at this dose level. | |||
Trovafloxacin was not mutagenic in the Ames Salmonella reversion assay or CHO/HGPRT mammalian cell gene mutation assay and it was not clastogenic in mitogen-stimulated human lymphocytes or mouse bone marrow cells. A mouse micronucleus test conducted with alatrofloxacin was also negative. The positive response observed in the E. coli bacterial mutagenicity assay may be due to the inhibition of DNA gyrase by trovafloxacin. | |||
|fdaPatientInfo=Patients should be advised: | |||
* to discontinue therapy and to inform their physician immediately if they develop symptoms suggestive of hepatic dysfunction including fatigue, anorexia, vomiting, abdominal pain, jaundice, dark urine or pale stool. | |||
* to inform their physician if they develop symptoms suggestive of pancreatitis including abdominal pain and/or nausea and vomiting. | |||
* that TROVAN Tablets may be taken without regard to meals; | |||
* that vitamins or minerals containing iron, aluminum- or magnesium-base antacids, antacids containing citric acid buffered with sodium citrate, or sucralfate or Videx®, (Didanosine), chewable/buffered tablets or the pediatric powder for oral solution, should be taken at least 2 hours before or 2 hours after taking TROVAN tablets; | |||
* that TROVAN may cause lightheadedness and/or dizziness. Dizziness and/or lightheadedness was the most common adverse reaction reported, and for females under 45 years, it was reported significantly more frequently than in other groups. The incidence of dizziness may be substantially reduced if TROVAN Tablets are taken at bedtime or with food. Patients should know how they react to trovafloxacin before they operate an automobile or machinery or engage in activities requiring mental alertness and coordination.; | |||
* to discontinue treatment and inform their physician if they experience pain, inflammation or rupture of a tendon, and to rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture has been confidently excluded; | |||
* that TROVAN may be associated with hypersensitivity reactions, even following the first dose, and to discontinue the drug at the first sign of a skin rash, hives or other skin reactions, difficulty in swallowing or breathing, any swelling suggesting angioedema (e.g., swelling of the lips, tongue, face, tightness of the throat, hoarseness), or other symptoms of an allergic reaction; | |||
* to avoid excessive sunlight or artificial ultraviolet light (e.g., tanning beds) while taking TROVAN and to discontinue therapy if phototoxicity (e.g., sunburn-like reaction or skin eruption) occurs. | |||
* that convulsions have been reported in patients taking quinolones, including trovafloxacin, and to notify their physician before taking this drug if there is a history of this condition. | |||
|alcohol=Alcohol-Trovafloxacin mesylate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Trovafloxacin mesylate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 16:48, 13 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Trovafloxacin mesylate is a fluoroquinolone that is FDA approved for the {{{indicationType}}} of {{{indication}}}. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Trovafloxacin mesylate FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Trovafloxacin mesylate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Trovafloxacin mesylate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Trovafloxacin mesylate FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Trovafloxacin mesylate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Trovafloxacin mesylate in pediatric patients.
Contraindications
Trovafloxacin mesylate is contraindicated in persons with a history of hypersensitivity to trovafloxacin, alatrofloxacin, quinolone antimicrobial agents or any other components of these products.
Warnings
Trovafloxacin mesylate-associated liver enzyme abnormalities, symptomatic hepatitis, jaundice and liver failure (including rare reports of acute hepatic necrosis with eosinophilic infiltration, liver transplantation and/or death) have been reported with both short-term and long-term drug exposure in men and women. Trovafloxacin use exceeding 2 weeks in duration is associated with a significantly increased risk of serious liver injury. Liver injury has also benn reportes following trovafloxacin re-exposure. clinicians should monitor liver function test (e.g., AST, ALT, bilirubin) in trovafloxacin recipients who develop signs or symptoms consistent with hepatitis. Clinicians should consider discontinuing travofloxacin in those patients who develop liver function test abnormalities.
The safety and effectiveness of trovafloxacin in pediatric patients and adolescents less than 18 years age, pregnant women, and nursing women have not been established.
As with other members of the quinolone class, trovafloxacin has caused arthropathy and/or chondrodysplasia in immature rats and dogs. The significance of these findings to humans is unknown. (See ANIMAL PHARMACOLOGY.)
Convulsions, increased intracranial pressure and psychosis have been reported in patients receiving quinolones. Quinolones may also cause central nervous system stimulation which may lead to tremors, restlessness, lightheadedness, confusion, hallucinations, paranoia, depression, nightmares and insomnia. These reactions may occur following the first dose. If these reactions occur in patients receiving trovafloxacin or alatrofloxacin, the drug should be discontinued and appropriate measures instituted. (See PRECAUTIONS: GENERAL,INFORMATION FOR PATIENTS, DRUG INTERACTIONS and ADVERSE REACTIONS.)
As with other quinolones, TROVAN should be used with caution in patients with known or suspected CNS disorders, such as severe cerebral atherosclerosis, epilepsy, and other factors that predispose to seizures. (See ADVERSE REACTIONS.)
Serious and occasionally fatal hypersensitivity and/or anaphylactic reactions have been reported in patients receiving therapy with TROVAN. These reactions may occur following the first dose. Some reactions have been accompanied by cardiovascular collapse, hypotension/shock, seizure, loss of consciousness, tingling, angioedema (including tongue, laryngeal, throat or facial edema/swelling), airway obstruction (including bronchospasm, shortness of breath and acute respiratory distress), dyspnea, urticaria, itching and other serious skin reactions, including generalized erythema.
Life-threatening hypotension has been reported with alatrofloxacin administration. This has occurred in patients receiving alatrofloxacin at either the recommended rate of infusion or if given more rapidly. Hypotension may be potentiated with the concomitant administration of anesthetic agents. Alatrofloxacin should only be administered by slow intravenous infusion over a period of 60 minutes. Blood pressure should be monitored closely during infusion.
TROVAN should be discontinued at the first appearance of a skin rash or any other sign of hypersensitivity. Serious acute hypersensitivity reactions may require treatment with epinephrine and other resuscitative measures, including oxygen, intravenous fluids, antihistamines, corticosteroids, pressor amines and airway management, as clinically indicated. (See PRECAUTIONS and ADVERSE REACTIONS.)
Serious and sometimes fatal events, some due to hypersensitivity and some due to uncertain etiology, have been reported in patients receiving therapy with all antibiotics. These events may be severe and generally occur following the administration of multiple doses. Clinical manifestations may include one or more of the following: fever, rash or severe dermatologic reactions (e.g., toxic epidermal necrolysis, Stevens-Johnson Syndrome); vasculitis, arthralgia, myalgia, serum sickness; allergic pneumonitis, interstitial nephritis; acute renal insufficiency or failure; hepatitis, jaundice, acute hepatic necrosis or failure; anemia, including hemolytic and aplastic; thrombocytopenia, including thrombotic thrombocytopenic purpura; leukopenia; agranulocytosis; pancytopenia; and/or other hematologic abnormalities.
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including TROVAN, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of any antibacterial agent.
Treatment with antibacterial agents alters the flora of the colon and may permit overgrowth of clostridia. Studies indicate that a toxin produced by Clostridium difficile is the primary cause of "antibiotic-associated colitis."
After the diagnosis of pseudomembranous colitis has been established, therapeutic measures should be initiated. Mild cases of pseudomembranous colitis usually respond to drug discontinuation alone. In moderate to severe cases, consideration should be given to management with fluids and electrolytes, protein supplementation, and treatment with an antibacterial drug clinically effective against C. difficile colitis. (See ADVERSE REACTIONS.)
Although not seen in TROVAN clinical trials, ruptures of the shoulder, hand, and Achilles tendons that required surgical repair or resulted in prolonged disability have been reported in patients receiving quinolones. TROVAN should be discontinued if the patient experiences pain, inflammation or rupture of a tendon. Patients should rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture has been confidently excluded. Tendon rupture can occur during or after therapy with quinolones.
Trovafloxacin has not been shown to be effective in the treatment of syphilis. Antimicrobial agents used in high doses for short periods of time to treat gonorrhea may mask or delay the symptoms of incubating syphilis. All patients with gonorrhea should have a serologic test for syphilis at the time of diagnosis.
Adverse Reactions
Clinical Trials Experience
Over 6000 patients have been treated with TROVAN in multidose clinical efficacy trials worldwide.
In TROVAN studies the majority of adverse reactions were described as mild in nature (over 90% were described as mild or moderate). TROVAN was discontinued for adverse events thought related to drug in 5% of patients (dizziness 2.4%, nausea 1.9%, headache 1.1%, and vomiting 1.0%).
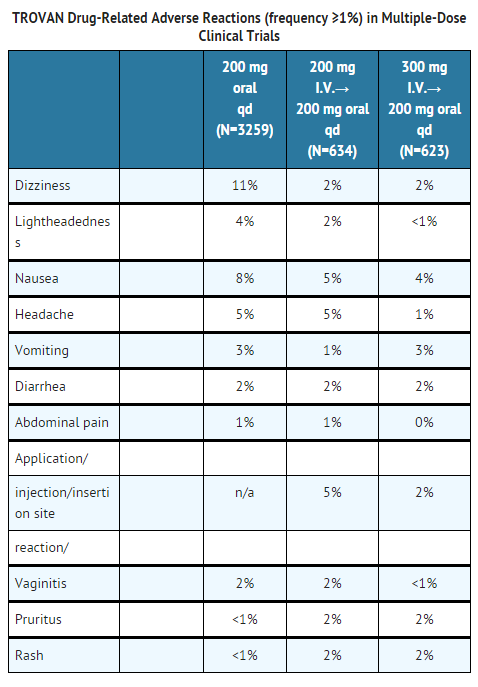
Dizziness/lightheadedness on TROVAN is generally mild, lasts for a few hours following a dose, and in most cases, resolves with continued dosing. The incidence of dizziness and lightheadedness in TROVAN patients over 65 years is 3.1% and 0.6%, respectively.
TROVAN appears to have a low potential for phototoxicity. In clinical trials with TROVAN, only mild, treatment-related phototoxicity was observed in less than 0.03% (2/7096) of patients.
Additional reported drug-related events in clinical trials (remotely, possibly, probably or unknown) that occurred in <1% of TROVAN-treated patients are:
APPLICATION/INJECTION/INSERTION SITE: Application/injection/insertion site device complications, inflammation, pain, edema
AUTONOMIC NERVOUS: flushing, increased sweating, dry mouth, cold clammy skin, increased saliva
CARDIOVASCULAR: peripheral edema, chest pain, thrombophlebitis, hypotension, palpitation, periorbital edema, hypertension, syncope, tachycardia, angina pectoris, bradycardia, peripheral ischemia, edema, dizziness postural
CENTRAL & PERIPHERAL NERVOUS SYSTEM: confusion, paresthesia, vertigo, hypoesthesia, ataxia, convulsions, dysphonia, hypertonia, migraine, involuntary muscle contractions, speech disorder, encephalopathy, abnormal gait, hyperkinesia, hypokinesia, tongue paralysis, abnormal coordination, tremor, dyskinesia
GASTROINTESTINAL: altered bowel habit, constipation, diarrhea-Clostridium difficile, dyspepsia, flatulence, loose stools, gastritis, dysphagia, increased appetite, gastroenteritis, rectal disorder, colitis, pseudomembranous colitis, enteritis, eructation, gastrointestinal disorder, melena, hiccup
ORAL CAVITY: gingivitis, stomatitis, altered saliva, tongue disorder, tongue edema, tooth disorder, cheilitis, halitosis
GENERAL/OTHER: fever, fatigue, pain, asthenia, moniliasis, hot flushes, back pain, chills, infection (bacterial, fungal), malaise, sepsis, alcohol intolerance, allergic reaction, anaphylactoid reaction, drug (other) toxicity/reaction, weight increase, weight decrease
HEMATOPOIETIC: anemia, granulocytopenia, hemorrhage unspecified, leukopenia, prothrombin decreased, thrombocythemia, thrombocytopenia
LIVER/BILIARY: increased hepatic enzymes, hepatic function abnormal, bilirubinemia, discolored feces, jaundice
METABOLIC/NUTRITIONAL: hyperglycemia, thirst
MUSCULOSKELETAL: arthralgia, muscle cramps, myalgia, muscle weakness, skeletal pain, tendinitis, arthropathy
PSYCHIATRIC: anxiety, anorexia, agitation, nervousness, somnolence, insomnia, depression, amnesia, concentration impaired, depersonalization, dreaming abnormal, emotional lability, euphoria, hallucination, impotence, libido decreased-male, paroniria, thinking abnormal
REPRODUCTIVE: Female: leukorrhea, menstrual disorder; Male: balanoposthitis
RESPIRATORY: dyspnea, rhinitis, sinusitis, bronchospasm, coughing, epistaxis, respiratory insufficiency, upper respiratory tract infection, respiratory disorder, asthma, hemoptysis, hypoxia, stridor
SKIN/APPENDAGES: pruritus ani, skin disorder, skin ulceration, angioedema, dermatitis, dermatitis fungal, photosensitivity skin reaction, seborrhea, skin exfoliation, urticaria
SPECIAL SENSES: taste perversion, eye pain, abnormal vision, conjunctivitis, photophobia, conjuctival hemorrhage, hyperacusis, scotoma, tinnitus, visual field defect, diplopia, xerophthalmia
URINARY SYSTEM: dysuria, face edema, micturition frequency, interstitial nephritis, renal failure acute, renal function abnormal, urinary incontinence
LABORATORY CHANGES: Changes in laboratory parameters, without regard to drug relationship, occurring in ≥1% of TROVAN-treated patients were: decreased hemoglobin and hematocrit; increased platelets; decreased and increased WBC; eosinophilia; increased ALT (SGPT), AST (SGOT), and alkaline phosphatase; decreased protein and albumin; increased BUN and creatinine; decreased sodium; and bicarbonate. It is not known whether these abnormalities were caused by the drug or the underlying condition being treated.
The incidence and magnitude of liver function abnormalities with TROVAN were the same as comparator agents except in the only study in which oral TROVAN was administered for 28 days. In this study (chronic bacterial prostatitis) nine percent (13/140) of TROVAN-treated patients experienced elevations of serum transaminases (AST and/or ALT) of ≥3 times the upper limit of normal. These liver function test abnormalities generally developed at the end of, or following completion of, the planned 28-day course of therapy, but were not associated with concurrent elevations of related laboratory measures of hepatic function (such as serum bilirubin, alkaline phosphatase, or lactate dehydrogenase). Patients were asymptomatic with these abnormalities, which generally returned to normal within 1–2 months after discontinuation of therapy.
Postmarketing Experience
Adverse reactions reported with TROVAN during the post-marketing period include:
- GASTROINTESTINAL: symptomatic pancreatitis.
- GENERAL/OTHER: anaphylaxis, Stevens-Johnson Syndrome.
- HEMATOPOIETIC: agranulocytosis, aplastic anemia, pancytopenia.
- LIVER/BILIARY: symptomatic hepatitis (some patients experienced an associated peripheral eosinophilia), liver failure (including acute hepatic necrosis with eosinophilic infiltration). TROVAN-associated liver enzyme abnormalities and/or symptomatic hepatitis have occurred during short-term or long-term therapy.
Drug Interactions
- Antacids, Sucralfate, and Iron: The absorption of oral trovafloxacin is significantly reduced by the concomitant administration of some antacids containing magnesium or aluminum, citric acid/sodium citrate (Bicitra®), as well as sucralfate and iron (ferrous ions). These agents as well as formulations containing divalent and trivalent cations such as Videx®, (Didanosine), chewable/buffered tablets or the pediatric powder for oral solution, should be taken at least 2 hours before or 2 hours after oral trovafloxacin administration.
- Morphine: Co-administration of intravenous morphine significantly reduces the absorption of oral trovafloxacin. Intravenous morphine should be administered at least 2 hours after oral TROVAN dosing in the fasted state and at least 4 hours after oral TROVAN is taken with food. Trovafloxacin administration had no effect on the pharmacokinetics of morphine or its metabolite, morphine-6-β-glucuronide.
- Warfarin: There have been reports during the post-marketing experience that trovafloxacin/alatrofloxacin enhance the effects of warfarin, including cases of bleeding. The mechanism for this reaction is unknown. Prothrombin time, International Normalized Ratio (INR) or other suitable anticoagulation tests should be closely monitored if trovafloxacin/alatrofloxacin is administered concomitantly with warfarin. Patients should also be monitored for evidence of bleeding.
- Minor pharmacokinetic interactions without clinical significance have been observed with co-administration of TROVAN Tablets with caffeine, omeprazole and calcium carbonate.
- No significant pharmacokinetic interactions with theophylline, cimetidine, digoxin, warfarin, or cyclosporine have been observed with TROVAN Tablets.
- Alatrofloxacin should not be co-administered with any solution containing multivalent cations, e.g., magnesium, through the same intravenous line.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C An increase in skeletal variations was observed in rat fetuses after daily oral 75 mg/kg maternal doses of trovafloxacin (approximately 15 times the highest recommended human dose based on mg/kg or 2 times based upon body surface area) were administered during organogenesis. However, fetal skeletal variations were not observed in rats dosed orally with 15 mg/kg trovafloxacin. Evidence of fetotoxicity (increased perinatal mortality and decreased body weights) was also observed in rats at 75 mg/kg. Daily oral doses of trovafloxacin at 45 mg/kg (approximately 9 times the highest recommended human dose based on mg/kg or 2.7 times based upon body surface area) in the rabbit were not associated with an increased incidence of fetal skeletal variations or malformations.
An increase in skeletal variations and malformations was observed in rat fetuses after daily intravenous doses of alatrofloxacin at ≥20 mg/kg/day (approximately 4 times the highest recommended human dose based on mg/kg or 0.6 times based upon body surface area) were administered to dams during organogenesis. In the rabbit, an increase in fetal skeletal malformations was also observed when 20 mg/kg/day (approximately equal to the highest recommended human dose based upon body surface area) of alatrofloxacin was given intravenously during the period of organogenesis. Intravenous dosing of alatrofloxacin at 6.5 mg/kg in the rat or rabbit was not associated with an increased incidence of skeletal variations or malformations. Fetotoxicity and fetal skeletal malformations have been associated with other quinolones.
Oral doses of trovafloxacin >5mg/kg were associated with an increased gestation time in rats, and several dams at 75 mg/kg experienced uterine dystocia.
There are no adequate and well-controlled studies in pregnant women. TROVAN should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Trovafloxacin mesylate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Trovafloxacin mesylate during labor and delivery.
Nursing Mothers
Trovafloxacin is excreted in human milk and was found in measurable concentrations in the breast milk of lactating subjects.
Because of the potential for unknown effects from trovafloxacin in nursing infants from mothers taking trovafloxacin, a decision should be made either to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of trovafloxacin in pediatric patients and adolescents less than 18 years of age have not been established. Quinolones, including trovafloxacin, cause arthropathy and osteochondrosis in juvenile animals of several species.
Geriatic Use
In multiple-dose clinical trials of trovafloxacin, 27% of patients were ≥65 years of age and 12% of patients were ≥75 years of age. The overall incidence of drug-related adverse reactions, including central nervous system and gastrointestinal side effects, was less in the ≥65 year group than the other age groups.
Gender
There are no significant differences in trovafloxacin pharmacokinetics between males and females when differences in body weight are taken into account. After single 200 mg doses, trovafloxacin Cmax and AUC(0–∞) were 60% and 32% higher, respectively, in healthy females compared to healthy males. Following repeated daily administration of 200 mg for 7 days, the Cmax for trovafloxacin was 38% higher and AUC(0–24) was 16% higher in healthy females compared to healthy males. The clinical importance of the increases in serum levels of trovafloxacin in females has not been established.
Race
There is no FDA guidance on the use of Trovafloxacin mesylate with respect to specific racial populations.
Renal Impairment
The pharmacokinetics of trovafloxacin are not affected by renal impairment. Trovafloxacin serum concentrations are not significantly altered in subjects with severe renal insufficiency (creatinine clearance <20 mL/min), including patients on hemodialysis.
Hepatic Impairment
Following repeated administration of 100 mg for 7 days to patients with mild cirrhosis (Child-Pugh Class A), the AUC(0–24) for trovafloxacin was increased ~45% compared to matched controls. Repeated administration of 200 mg for 7 days to patients with moderate cirrhosis (Child-Pugh Class B) resulted in an increase of ~50% in AUC(0–24) compared to matched controls. There appeared to be no significant effect on trovafloxacin Cmax for either group. The oral clearance of trovafloxacin was reduced ~30% in both cirrhosis groups, which corresponded to prolongation of half life by 2–2.5 hours (25–30% increase) compared to controls. There are no data in patients with severe cirrhosis (Child-Pugh Class C). Dosage adjustment is recommended in patients with mild to moderate cirrhosis.
Females of Reproductive Potential and Males
Trovafloxacin and alatrofloxacin did not affect the fertility of male or female rats at oral and I.V. doses of 75 mg/kg/day and 50 mg/kg/day, respectively. These doses are 15 and 10 times the recommended maximum human dose based on mg/kg or approximately 2 times based on mg/m2. However, oral doses of trovafloxacin at 200 mg/kg/day (40 times the recommended maximum human dose based on mg/kg or about 6 times based on mg/m2) were associated with increased preimplantation loss in rats.
Immunocompromised Patients
There is no FDA guidance one the use of Trovafloxacin mesylate in patients who are immunocompromised.
Photosensitivity Potential
In a study of the skin response to ultraviolet and visible radiation conducted in 48 healthy volunteers (12 per group), the minimum erythematous dose (MED) was measured for ciprofloxacin, lomefloxacin, trovafloxacin and placebo before and after drug administration for 5 days. In this study, trovafloxacin (200 mg q.d.) was shown to have a lower potential for producing delayed photosensitivity skin reactions than ciprofloxacin (500 mg b.i.d.) or lomefloxacin (400 mg q.d.), although greater than placebo.
Administration and Monitoring
Administration
Intravenous
Monitoring
There is limited information regarding Trovafloxacin mesylate Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Trovafloxacin mesylate and IV administrations.
Overdosage
There is limited information regarding Trovafloxacin mesylate overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Trovafloxacin mesylate Pharmacology in the drug label.
Mechanism of Action
Trovafloxacin is a fluoronaphthyridone related to the fluoroquinolones with in vitro activity against a wide range of gram-negative and gram-positive aerobic, and anaerobic microorganisms. The bactericidal action of trovafloxacin results from inhibition of DNA gyrase and topoisomerase IV. DNA gyrase is an essential enzyme that is involved in the replication, transcription and repair of bacterial DNA. Topoisomerase IV is an enzyme known to play a key role in the partitioning of the chromosomal DNA during bacterial cell division. Mechanism of action of fluoroquinolones including trovafloxacin is different from that of penicillins, cephalosporins, aminoglycosides, macrolides, and tetracyclines. Therefore, fluoroquinolones may be active against pathogens that are resistant to these antibiotics. There is no cross-resistance between trovafloxacin and the mentioned classes of antibiotics. The overall results obtained from in vitro synergy studies, testing combinations of trovafloxacin with beta-lactams and aminoglycosides, indicate that synergy is strain specific and not commonly encountered. This agrees with results obtained previously with other fluoroquinolones. Resistance to trovafloxacin in vitro develops slowly via multiple-step mutation in a manner similar to other fluoroquinolones. Resistance to trovafloxacin in vitro occurs at a general frequency of between 1×10-7 to 10-10. Although cross-resistance has been observed between trovafloxacin and some other fluoroquinolones, some microorganisms resistant to other fluoroquinolones may be susceptible to trovafloxacin.
Structure
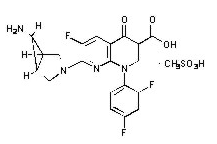
The chemical structure is:
Pharmacodynamics
There is limited information regarding Trovafloxacin mesylate Pharmacodynamics in the drug label.
Pharmacokinetics
After intravenous administration, alatrofloxacin is rapidly converted to trovafloxacin. Plasma concentrations of alatrofloxacin are below quantifiable levels within 5 to 10 minutes of completion of a 1 hour infusion.
Absorption
Trovafloxacin is well-absorbed from the gastrointestinal tract after oral administration. The absolute bioavailability is approximately 88%. For comparable dosages, no dosage adjustment is necessary when switching from parenteral to oral administration.
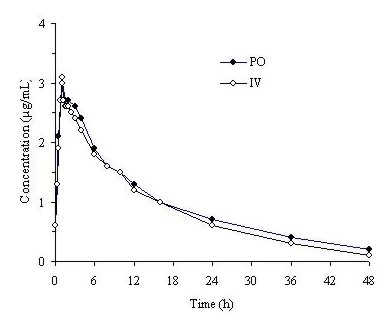
Figure 1. Mean trovafloxacin serum concentrations determined following 1 hour intravenous infusions of alatrofloxacin at daily doses of 200 mg (trovafloxacin equivalents) to healthy male volunteers and following daily oral administration of 200 mg trovafloxacin for 7 days to six male and six female healthy young volunteers.
Pharmacokinetics Parameters
The mean pharmacokinetic parameters (±SD) of trovafloxacin after single and multiple 100 mg and 200 mg oral doses and 1 hour intravenous infusions of alatrofloxacin in doses of 200 and 300 mg (trovafloxacin equivalents) appear in the chart below.
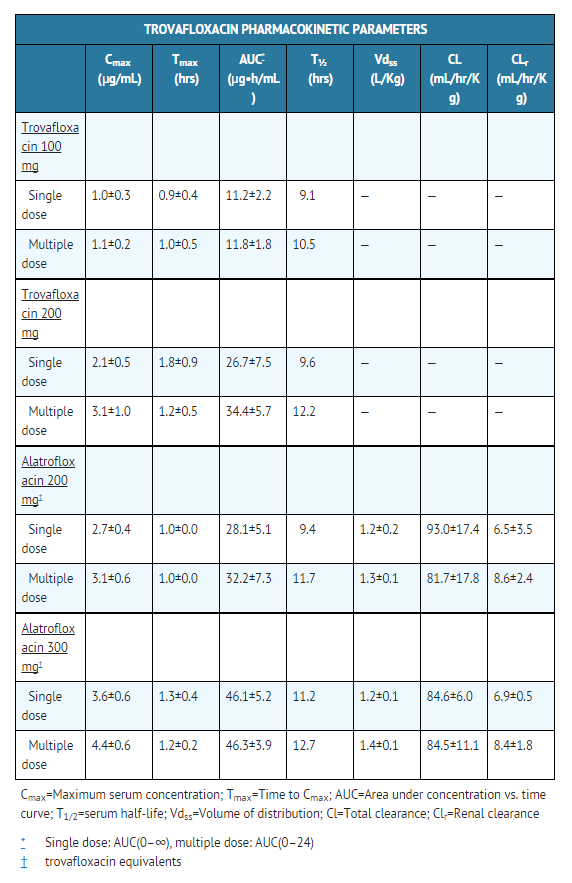
Serum concentrations of trovafloxacin are dose proportional after oral administration of trovafloxacin in the dose range of 30 to 1000 mg or after intravenous administration of alatrofloxacin in the dose range of 30 to 400 mg (trovafloxacin equivalents). Steady state concentrations are achieved by the third daily oral or intravenous dose of trovafloxacin with an accumulation factor of approximately 1.3 times the single dose concentrations.
Oral absorption of trovafloxacin is not altered by concomitant food intake; therefore, it can be administered without regard to food.
The systemic exposure to trovafloxacin (AUC0–∞) administered as crushed tablets via nasogastric tube into the stomach was identical to that of orally administered intact tablets. Administration of concurrent enteral feeding solutions had no effect on the absorption of trovafloxacin given via nasogastric tube into the stomach. When trovafloxacin was administered as crushed tablets into the duodenum via nasogastric tube, the AUC0–∞ and peak serum concentration (Cmax) were reduced by 30% relative to the orally administered intact tablets. Time to peak serum level (Tmax) was also decreased from 1.7 hrs to 1.1 hrs.
Distribution
The mean plasma protein bound fraction is approximately 76%, and is concentration-independent. Trovafloxacin is widely distributed throughout the body. Rapid distribution of trovafloxacin into tissues results in significantly higher trovafloxacin concentrations in most target tissues than in plasma or serum.
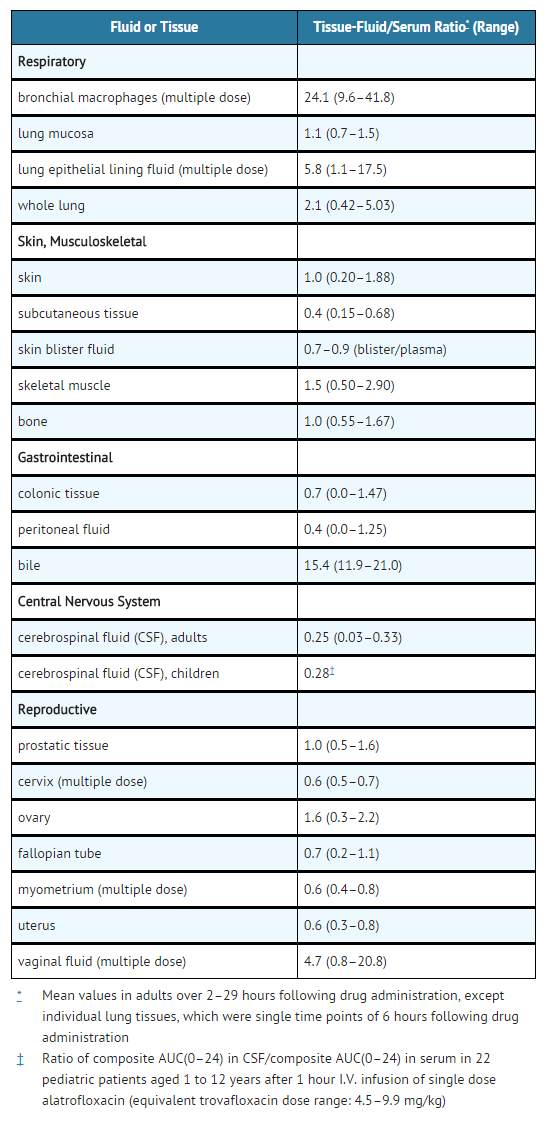
Presence in Breast Milk
Trovafloxacin was found in measurable concentrations in the breast milk of three lactating subjects. The average measurable breast milk concentration was 0.8 µg/mL (range: 0.3–2.1 µg/mL) after single I.V. alatrofloxacin (300 mg trovafloxacin equivalents) and repeated oral trovafloxacin (200 mg) doses.
Metabolism
Trovafloxacin is metabolized by conjugation (the role of cytochrome P450 oxidative metabolism of trovafloxacin is minimal). Thirteen percent of the administered dose appears in the urine in the form of the ester glucuronide and 9% appears in the feces as the N-acetyl metabolite (2.5% of the dose is found in the serum as the active N-acetyl metabolite). Other minor metabolites (diacid, sulfamate, hydroxycarboxylic acid) have been identified in both urine and feces in small amounts (<4% of the administered dose).
Excretion
Approximately 50% of an oral dose is excreted unchanged (43% in the feces and 6% in the urine).
After multiple 200 mg doses, to healthy subjects, mean (±SD) cumulative urinary trovafloxacin concentrations were 12.1±3.4 µg/mL. With these levels of trovafloxacin in urine, crystals of trovafloxacin have not been observed in the urine of human subjects.
Nonclinical Toxicology
Carcinogenesis and Mutagenesis
Long term studies in animals to determine the carcinogenic potential of trovafloxacin or alatrofloxacin have not been conducted.
TROVAN did not shorten the time to development of UV-induced skin tumors in hairless albino (Skh-1) mice; thus, it was not photo co-carcinogenic in this model. These mice received oral trovafloxacin and concurrent irradiation with simulated sunlight 5 days per week for 40 weeks followed by a 12-week treatment-free observation period. The daily dose of UV radiation used in this study was approximately 30% of the minimal dose of UV radiation that would induce erythema in Caucasian humans. The median time to the development of skin tumors in the hairless mice (42–43 weeks) was similar in the vehicle control group and those given 10 or 30 mg/kg of trovafloxacin daily. At a dose level of 30 mg/kg/day, the mice had skin trovafloxacin concentrations of approximately 7 µg/g. Following multiple 200 mg daily doses of trovafloxacin, the amount in human skin is estimated to be about 3 µg/g, based upon plasma concentrations measured at this dose level.
Trovafloxacin was not mutagenic in the Ames Salmonella reversion assay or CHO/HGPRT mammalian cell gene mutation assay and it was not clastogenic in mitogen-stimulated human lymphocytes or mouse bone marrow cells. A mouse micronucleus test conducted with alatrofloxacin was also negative. The positive response observed in the E. coli bacterial mutagenicity assay may be due to the inhibition of DNA gyrase by trovafloxacin.
Clinical Studies
There is limited information regarding Trovafloxacin mesylate Clinical Studies in the drug label.
How Supplied
There is limited information regarding Trovafloxacin mesylate How Supplied in the drug label.
Storage
There is limited information regarding Trovafloxacin mesylate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Trovafloxacin mesylate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Trovafloxacin mesylate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Patients should be advised:
- to discontinue therapy and to inform their physician immediately if they develop symptoms suggestive of hepatic dysfunction including fatigue, anorexia, vomiting, abdominal pain, jaundice, dark urine or pale stool.
- to inform their physician if they develop symptoms suggestive of pancreatitis including abdominal pain and/or nausea and vomiting.
- that TROVAN Tablets may be taken without regard to meals;
- that vitamins or minerals containing iron, aluminum- or magnesium-base antacids, antacids containing citric acid buffered with sodium citrate, or sucralfate or Videx®, (Didanosine), chewable/buffered tablets or the pediatric powder for oral solution, should be taken at least 2 hours before or 2 hours after taking TROVAN tablets;
- that TROVAN may cause lightheadedness and/or dizziness. Dizziness and/or lightheadedness was the most common adverse reaction reported, and for females under 45 years, it was reported significantly more frequently than in other groups. The incidence of dizziness may be substantially reduced if TROVAN Tablets are taken at bedtime or with food. Patients should know how they react to trovafloxacin before they operate an automobile or machinery or engage in activities requiring mental alertness and coordination.;
- to discontinue treatment and inform their physician if they experience pain, inflammation or rupture of a tendon, and to rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture has been confidently excluded;
- that TROVAN may be associated with hypersensitivity reactions, even following the first dose, and to discontinue the drug at the first sign of a skin rash, hives or other skin reactions, difficulty in swallowing or breathing, any swelling suggesting angioedema (e.g., swelling of the lips, tongue, face, tightness of the throat, hoarseness), or other symptoms of an allergic reaction;
- to avoid excessive sunlight or artificial ultraviolet light (e.g., tanning beds) while taking TROVAN and to discontinue therapy if phototoxicity (e.g., sunburn-like reaction or skin eruption) occurs.
- that convulsions have been reported in patients taking quinolones, including trovafloxacin, and to notify their physician before taking this drug if there is a history of this condition.
Precautions with Alcohol
Alcohol-Trovafloxacin mesylate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Trovafloxacin mesylate Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Trovafloxacin mesylate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.