Trimipramine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Pratik Bahekar, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Suicidality and Antidepressant Drugs
See full prescribing information for complete Boxed Warning.
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of trimipramine maleate or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Trimipramine maleate is not approved for use in pediatric patients.
|
Overview
Trimipramine is a Tricyclic antidepressant that is FDA approved for the {{{indicationType}}} of depression. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hypotension, tachycardia, constipation, xerostomia, dizziness, somnolence, blurred vision.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Depression
- 75 mg PO (divided into 1-3 doses per day);
- Increase gradually over a few days to 150 mg/day (in divided doses); max dosage is 200 mg/day
- Maintenance, 50-150 mg/day PO at bedtime; therapy should be continued for about 3 months
- Inpatients, 100 mg PO (divided into 1-3 doses per day); increase gradually over a few days to 200 mg/day (in divided doses);
- If no improvement in 2-3 weeks may increase to a max of 250-300 mg/day
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Trimipramine in adult patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Trimipramine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Depression
- Not FDA-approved for use in children
- Adolescents, 50 mg/day PO increase up to 100 mg/day;
- Maintenance therapy should be continued for about 3 months.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Trimipramine in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Trimipramine in pediatric patients.
Contraindications
- Monoamine Oxidase Inhibitors (MAOIs)
- The use of MAOIs intended to treat psychiatric disorders with trimipramine maleate or within 14 days of stopping treatment with trimipramine maleate is contraindicated because of an increased risk of serotonin syndrome. The use of trimipramine maleate within 14 days of stopping an MAOI intended to treat psychiatric disorders is also contraindicated.
- Starting trimipramine maleate in a patient who is being treaetd with MAOIs such as linezolid or intravenous methylene blue is also contraindicated because of an increased risk of serotonin syndrome.
- Hypersensitivity to Tricyclic Antidepressants
- Cross-sensitivity between trimipramine maleate and other dibenzazepines is a possibility.
- Myocardial Infarction
- The drug is contraindicated during the acute recovery period after a myocardial infarction.
Warnings
|
Suicidality and Antidepressant Drugs
See full prescribing information for complete Boxed Warning.
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of trimipramine maleate or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Trimipramine maleate is not approved for use in pediatric patients.
|
- Clinical Worsening and Suicide Risk
- Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (aged 18-24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analysis of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD) or other psychiatric disorders including a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients.
- There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs placebo), however, were relatively stable with age strada and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 1.

- No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression. All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
- The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient’s presenting symptoms. Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and non-psychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for trimipramine maleate should be written for the smallest quantity of capsules consistent with good patient management, in order to reduce the risk of overdose.
- Screening Patients for Bipolar Disorder: A major depressive episode may be the initial presentation of bipolar disorder. It is generally believed (though not established in controlled trials) that treating such an episode with an antidepressant alone may increase the likelihood of precipitation of a mixed/manic episode in patients at risk for bipolar disorder. Whether any of the symptoms described above represent such a conversion is unknown. However, prior to initiating treatment with an antidepressant, patients with depression symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression. It should be noted that trimipramine maleate is not approved for use in treating bipolar depression.
- Serotonin Syndrome
- The development of a potentially life-threatening serotonin syndrome has been reported with SNRIs and SSRIs, including trimipramine maleate, alone, but particularly with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, and St. John’s Wort) and with drugs that impair metabolism of serotonin (in particular, MAOIs, both those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue).
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g, nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotonin syndrome.
- The concomitant use of trimipramine maleate with MAOIs intended to treat psychiatric disorders is contraindicated. Trimipramine maleate should also not be started in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue. All reports with methylene blue that provided information on the route of administration involved intravenous administration in the dose range of 1 mg/kg to 8 mg/kg. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection) or at lower doses. There may be circumstances when it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking trimipramine maleate. Trimipramine maleate should be discontinued before initiating treatment with the MAOI.
- If concomitant use of trimipramine maleate with other serotonergic drugs, including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, buspirone, tryptophan, and St. John’s Wort is clinically warranted, patients should be made aware of a potential increased risk for serotonin syndrome, particularly during treatment initiation and dose increases.
Treatment with trimipramine maleate and any concomitant serotonergic agents should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated.
- General Consideration for Use
- Extreme caution should be used when this drug is given to patients with any evidence of cardiovascular disease because of the possibility of conduction defects, arrhythmias, myocardial infarction, strokes, and tachycardia.
Caution is advised in patients with increased intraocular pressure, history of urinary retention, or history of narrow-angle glaucoma because of the drug’s anticholinergic properties; hyperthyroid patients or those on thyroid medication because of the possibility of cardiovascular toxicity; patients with a history of seizure disorder, because this drug has been shown to lower the seizure threshold; patients receiving guanethidine or similar agents, since trimipramine maleate may block the pharmacologic effects of these drugs. Since the drug may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks, such as operating an automobile or machinery, the patient should be cautioned accordingly.
- General
- The possibility of suicide is inherent in any severely depressed patient and persists until a significant remission occurs. When a patient with a serious suicidal potential is not hospitalized, the prescription should be for the smallest amount feasible.
In schizophrenic patients activation of the psychosis may occur and require reduction of dosage or the addition of a major tranquilizer to the therapeutic regime.
- Manic or hypomanic episodes may occur in some patients, in particular those with cyclic-type disorders. In some cases therapy with trimipramine maleate must be discontinued until the episode is relieved, after which therapy may be reinstituted at lower dosages if still required.
Concurrent administration of trimipramine maleate and electroshock therapy may increase the hazards of therapy. Such treatment should be limited to those patients for whom it is essential. When possible, discontinue the drug for several days prior to elective surgery. Trimipramine maleate should be used with caution in patients with impaired liver function. Chronic animal studies showed occasional occurrence of hepatic congestion, fatty infiltration, or increased serum liver enzymes at the highest dose of 60 mg/kg/day.
- Both elevation and lowering of blood sugar have been reported with tricyclic antidepressants.
- Information for Patients
- Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with trimipramine maleate and should counsel them in its appropriate use. A patient Medication Guide about “Antidepressant Medicines, Depression and other Serious Mental Illness, and Suicidal Thoughts or Actions” is available for trimipramine maleate. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
- Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking trimipramine maleate.
- Clinical Worsening and Suicide Risk:
- Patients, their families, and their caregivers should be encouraged to be alert to the emergence of anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia, (psychomotor restlessness), hypomania, mania, other unusual changes in behavior, worsening of depression, and suicidal ideation, especially early during antidepressant treatment and when the dose is adjusted up or down. Families and caregivers of patients should be advised to look for the emergence of such symptoms on a day-to-day basis, since changes may be abrupt. Such symptoms should be reported to the patient’s prescriber or health professional, especially if they are severe, abrupt in onset, or were not part of the patient’s presenting symptoms. Symptoms such as these may be associated with an increased risk for suicidal thinking and behavior and indicate a need for very close monitoring and possibly changes in the medication.
Adverse Reactions
Clinical Trials Experience
Note: The pharmacological similarities among the tricyclic antidepressants require that each of the reactions be considered when trimipramine maleate is administered. Some of the adverse reactions included in this listing have not in fact been reported with trimipramine maleate.
- Cardiovascular
- Hypotension, hypertension, tachycardia, palpitation, myocardial infarction, arrhythmias, heart block, stroke.
- Psychiatric
- Confusional states (especially the elderly) with hallucinations, disorientation, delusions; anxiety, restlessness, agitation; insomnia and nightmares; hypomania; exacerbation of psychosis.
- Neurological
- Numbness, tingling, paresthesias of extremities; incoordination, ataxia, tremors; peripheral neuropathy; extrapyramidal symptoms; seizures, alterations in EEG patterns; tinnitus; syndrome of inappropriate ADH (antidiuretic hormone) secretion.
- Anticholinergic
- Dry mouth and, rarely, associated sublingual adenitis; blurred vision, disturbances of accommodation, mydriasis, constipation, paralytic ileus; urinary retention, delayed micturition, dilation of the urinary tract.
- Allergic
- Skin rash, petechiae, urticaria, itching, photosensitization, edema of face and tongue.
- Hematologic
- Bone-marrow depression including agranulocytosis, eosinophilia; purpura; thrombocytopenia. Leukocyte and differential counts should be performed in any patient who develops fever and sore throat during therapy; the drug should be discontinued if there is evidence of pathological neutrophil depression.
- Gastrointestinal
- Nausea and vomiting, anorexia, epigastric distress, diarrhea, peculiar taste, stomatitis, abdominal cramps, black tongue.
- Endocrine
- Gynecomastia in the male; breast enlargement and galactorrhea in the female; increased or decreased libido, impotence; testicular swelling; elevation or depression of blood-sugar levels.
- Other
- Jaundice (simulating obstructive); altered liver function; weight gain or loss; perspiration; flushing; urinary frequency; drowsiness, dizziness, weakness, and fatigue; headache; parotid swelling; alopecia.
- Withdrawal Symptoms
- Though not indicative of addiction, abrupt cessation of treatment after prolonged therapy may produce nausea, headache, and malaise.
Postmarketing Experience
There is limited information regarding Trimipramine Postmarketing Experience in the drug label.
Drug Interactions
- Cimetidine
- There is evidence that cimetidine inhibits the elimination of tricyclic antidepressants. Downward adjustment of trimipramine maleate dosage may be required if cimetidine therapy is initiated; upward adjustment if cimetidine therapy is discontinued.
- Alcohol
- Patients should be warned that the concomitant use of alcoholic beverages may be associated with exaggerated effects.
Catecholamines/Anticholinergics It has been reported that tricyclic antidepressants can potentiate the effects of catecholamines. Similarly, atropine-like effects may be more pronounced in patients receiving anticholinergic therapy. Therefore, particular care should be exercised when it is necessary to administer tricyclic antidepressants with sympathomimetic amines, local decongestants, local anesthetics containing epinephrine, atropine or drugs with an anticholinergic effect. In resistant cases of depression in adults, a dose of 2.5 mg/kg/day may have to be exceeded. If a higher dose is needed, ECG monitoring should be maintained during the initiation of therapy and at appropriate intervals during stabilization of dose.
- Drugs Metabolized by P450 2D6
- The biochemical activity of the drug metabolizing isozyme cytochrome P450 2D6 (debrisoquin hydroxylase) is reduced in a subset of the caucasian population (about 7-10% of caucasians are so called “poor metabolizers”); reliable estimates of the prevalence of reduced P450 2D6 isozyme activity among Asian, African, and other populations are not yet available. Poor metabolizers have higher than expected plasma concentrations of tricyclic antidepressants (TCAs) when given usual doses. Depending on the fraction of drug metabolized by P450 2D6, the increase in plasma concentration may be small, or quite large (8 fold increase in plasma AUC of the TCA).
- In addition, certain drugs inhibit the activity of the isozyme and make normal metabolizers resemble poor metabolizers. An individual who is stable on a given dose of TCA may become abruptly toxic when given one of these inhibiting drugs as concomitant therapy. The drugs that inhibit cytochrome P450 2D6 include some that are not metabolized by the enzyme (quinidine; cimetidine) and many that are substrates for P450 2D6 (many other antidepressants, phenothiazines, and the Type 1C antiarrhythmics propafenone and flecainide). While all the selective serotonin reuptake inhibitors (SSRIs), e.g., fluoxetine, sertraline, and paroxetine, inhibit P450 2D6, they may vary in the extent of inhibition.
The extent to which SSRI TCA interactions may pose clinical problems will depend on the degree of inhibition and the pharmacokinetics of the SSRI involved. Nevertheless, caution is indicated in the co-administration of TCAs with any of the SSRIs and also in switching from one class to the other. Of particular importance, sufficient time must elapse before initiating TCA treatment in a patient being withdrawn from fluoxetine, given the long half-life of the parent and active metabolite (at least 5 weeks may be necessary).
- Concomitant use of tricyclic antidepressants with drugs that can inhibit cytochrome P450 2D6 may require lower doses than usually prescribed for either the tricyclic antidepressant or the other drug. Furthermore, whenever one of these other drugs is withdrawn from co-therapy, an increased dose of tricyclic antidepressant may be required. It is desirable to monitor TCA plasma levels whenever a TCA is going to be co-administered with another drug known to be an inhibitor of P450 2D6.
- Monoamine Oxidase Inhibitors (MAOIs)
- Serotonergic Drugs
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Semen studies in man (four schizophrenics and nine normal volunteers) revealed no significant changes in sperm morphology. It is recognized that drugs having a parasympathetic effect, including tricyclic antidepressants, may alter the ejaculatory response.
- Chronic animal studies showed occasional evidence of degeneration of seminiferous tubules at the highest dose of 60 mg/kg/day.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C
Trimipramine maleate has shown evidence of embryotoxicity and/or increased incidence of major anomalies in rats or rabbits at doses 20 times the human dose. There are no adequate and well-controlled studies in pregnant women. Trimipramine maleate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Trimipramine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Trimipramine during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Trimipramine in women who are nursing.
Pediatric Use
Safety and effectiveness in the pediatric population have not been established. Anyone considering the use of trimipramine maleate in a child or adolescent must balance the potential risks with the clinical need.
Geriatic Use
Clinical studies of trimipramine maleate were not adequate to determine whether subjects aged 65 and over respond differently from younger subjects. The pharmacokinetics of trimipramine maleate was not substantially altered in the elderly. Trimipramine maleate is known to be substantially excreted by the kidney. Clinical circumstances, some of which may be more common in the elderly, such as hepatic or renal impairment, should be considered. Greater sensitivity (e.g., confusional states, sedation) of some older individuals cannot be ruled out. In general, dose selection for an elderly patient should be cautious, usually starting at a lower dose.
Gender
There is no FDA guidance on the use of Trimipramine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Trimipramine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Trimipramine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Trimipramine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Trimipramine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Trimipramine in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Trimipramine Administration in the drug label.
Monitoring
There is limited information regarding Trimipramine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Trimipramine and IV administrations.
Overdosage
There is limited information regarding Trimipramine overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Mechanism of Action
Trimipramine maleate is an antidepressant with an anxiety-reducing sedative component to its action. The mode of action of trimipramine maleate on the central nervous system is not known. However, unlike amphetamine-type compounds it does not act primarily by stimulation of the central nervous system. It does not act by inhibition of the monoamine oxidase system. The single-dose pharmacokinetics of trimipramine was evaluated in a comparative study of 24 elderly subjects and 24 younger subjects; no clinically relevant differences were demonstrated based on age or gender.
Structure
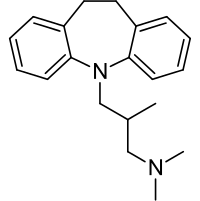
Trimipramine maleate is 5-(3-dimethylamino-2-methylpropyl)-10, 11-dihydro-5H-dibenz (b,f) azepine acid maleate (racemic form).
Trimipramine maleate capsules contain trimipramine maleate equivalent to 25 mg, 50 mg or 100 mg of trimipramine as the base. Inactive Ingredients: Each capsule contains lactose monohydrate and magnesium stearate. The capsule shell contains the following ingredients: D&C Yellow 10 (25 mg and 50 mg), FD&C Blue #1 (25 mg, 50 mg and 100 mg), FD&C Red #40 (50 mg), gelatin, and titanium dioxide. The capsules are imprinted in black ink that contains: alcohol, D&C yellow No.10 aluminum lake, FD&C blue No. 2/indigo carmine aluminum lake, FD&C blue No. 1/brilliant blue FCF aluminum lake, FD&C red No. 40/allura red AC aluminum lake, propylene glycol, iron oxide black and shellac glaze. Trimipramine maleate is prepared as a racemic mixture which can be resolved into levorotatory and dextrorotatory isomers. The asymmetric center responsible for optical isomerism is marked in the formula by an asterisk. Trimipramine maleate is an almost odorless, white or slightly cream-colored, crystalline substance, melting at 140°-144°C. It is very slightly soluble in ether and water, is slightly soluble in ethyl alcohol and acetone, and freely soluble in chloroform and methanol at 20°C.
Pharmacodynamics
There is limited information regarding Trimipramine Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Trimipramine Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Trimipramine Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Trimipramine Clinical Studies in the drug label.
How Supplied
There is limited information regarding Trimipramine How Supplied in the drug label.
Storage
There is limited information regarding Trimipramine Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Trimipramine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Trimipramine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Trimipramine Patient Counseling Information in the drug label.
Precautions with Alcohol
Patients should be warned that the concomitant use of alcoholic beverages may be associated with exaggerated effects.
Brand Names
There is limited information regarding Trimipramine Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Trimipramine Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 1.2 1.3 1.4 "PRODUCT INFORMATION SURMONTIL® Tablets and Capsules" (PDF). TGA eBusiness Services. Aspen Pharmacare Australia Pty Ltd. 28 November 2012. Retrieved 30 November 2013.
- ↑ 2.0 2.1 2.2 2.3 2.4 "SURMONTIL (trimipramine maleate) capsule [Duramed Pharmaceuticals Inc]". DailyMed. Duramed Pharmaceuticals Inc. December 2012. Retrieved 30 November 2013.
- ↑ 3.0 3.1 3.2 3.3 3.4 "Surmontil, Trimip (trimipramine) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 30 November 2013.
- ↑ 4.0 4.1 4.2 4.3 4.4 "Trimipramine 50mg Capsules - Summary of Product Characteristics (SPC)". electronic Medicines Compendium. Zentiva. 19 November 2012. Retrieved 30 November 2013.