Transcatheter aortic valve replacement
Editors-in-Chief: Roger Laham, M.D., C. Michael Gibson, M.S., M.D.; Jeffrey J. Popma, M.D.
Assistant Editor-In-Chief: Saleh El Dassouki, MD [1]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Synonyms and keywords: TAVI, Edward's valve, Edward's SAPIEN transcatheter heart valve, CoreValve, percutaneous aortic valve replacement, PAVR
Overview
Untill recently, aortic valve replacement (AVR) was the only effective treatment to severe symptomatic aortic stenosis. However, over the past decade percutaneous treatment of aortic valve disease with implantation of a stent-based valve prosthesis has been introduced as a new treatment in patients considered inoperable because of severe co-morbidities.[1] In Transcatheter Aortic Valve Implantation (TAVI) also known as Percutaneous Aortic Valve Replacement (PAVR), a synthetic valve is transported to the heart through a small hole made in groin. This procedure can be compared to that performed when placing a stent, or performing balloon angioplasty. Traditional aortic valve replacement is an invasive surgical procedure, with considerable mortality and morbidity, especially in more fragile patients. In the newly developed TAVI procedure, the dysfunctional aortic valve is replaced percutaneously, which removes the need for open heart surgery.
Valve types
CoreValve
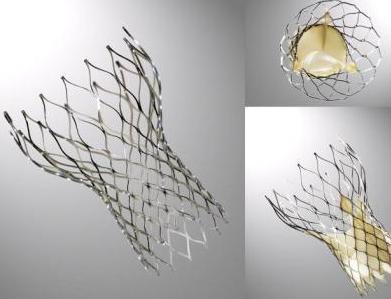
The CoreValve device was first inserted in 2005[1].[2]It consists of three leaflets of bioprosthetic pericardial tissue valve mounted on a self-expendable nitinol stent, which expands from the left ventricular outflow tract (LVOT) to the ascending aorta. The CoreValve frame is currently available in two sizes;a 26-mm design for aortic annular sizes between 20 and 23 mm and a 29-mm design for aortic annular sizes between 23 and 27-mm. The multilevel nitinol frame was designed for optimal functionality,stability,and durability. The inflow portion of the frame exerts high radial expansive force to provide proper support of the frame within the annular location.[3] The design of this portion of the frame prevents annular recoil, allowing the frame to partially conform to the non circular shape of the aortic annulus. The center portion of the frame has very high hoop strength that resists size and shape deformation which is a very important part of the device since it contains the valve leaflets, which are supra-annular.This center portion of the frame is concave to allow normal flow of blood through the coronaries and coronary cannulation after implantation. The largest part of the frame is the outflow portion that exerts low radial forces and allow optimal flow of blood through the valve. Porcine pericardium was selected due to its lower profile(compared with bovine pericardium)and its durability. The trileaflet valve is made of six individual pieces of porcine pericardium, with three pieces used to make a skirt at the inflow section of the valve thus preventing aortic regurgitation and three leaflet elements that are constructed with long commissures to distribute the aortic pressure load to the valve leaflets and the commissural posts.
The Edwards SAPIEN valve
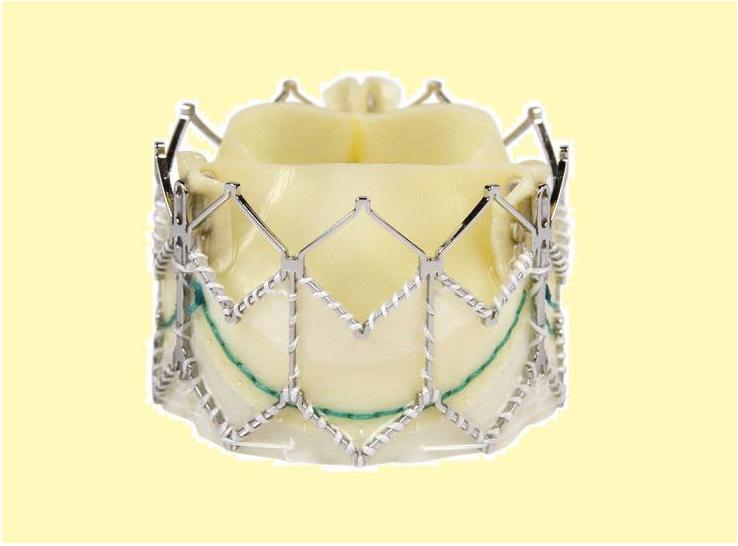
This prosthesis is considered the second generation of the Cribier-Edwards valve[4]. It is a balloon-expendable valve. made of a stainless steel frame covered by a Dacron skirt where three leaflets of pericardium are sutured. The device is placed in a subcoronary position during rapid ventricular pacing, via anterograde transapical or retrograde transfemoral approach. It is available in two sizes(23mm and 26 mm). In the first generation the leaflets were made of equine pericardium; in the second generation they are made of bovine pericardium with big improvements in the frame suture and an increase in the skirt length to decrease the risk of aortic regurgitation
Patient selection
Patient selection is very important before the performation of transcatheter aortic valve implant (TAVI). Candidates considered for TAVI must have severe aortic stenosis with contraindication to surgery. The patients selected for this procedure should have a potential for functional improvement after valve replacement.[5] Selection criteria depend on thorough evaluation of the aortic valve, mitral valve, ejection fraction, vascular access and coronary angiography. An echocardiographic investigation of the aortic valve is necessary as well, to determine the valve area, peak velocity. transvalvular gradient and aortic annulus. Aortic incompetence should be assessed as well since more than moderate aortic incompetence before the procedure is a contraindication for TAVI. Tortuosity, calcification and minimal luminal diameter of the aorta, iliac and femoral arteries would also influence patient selection and the technical approach used during the procedure.
Procedure
The diseased valve is moved aside by Aortic balloon valvuloplasty, than the Corevalve prosthesis is loaded in a specialized delivery catheter and advanced to the stenosed aortic valve. Once correctly positioned, the external part of the delivery system is progressively retracted, deploying the Corevalve Prosthesis. The delivery catheter is then closed and retrieved. <youtube v=7EhoUbWHW2A/>
Techniques
Two major catheter based techniques for replacing the aortic valve have been investigated [6]: retrograde percutaneous implantation and direct apical puncture. An antegrade transseptal approach has also been studied but not fully adopted.
- Retrograde approach[7]: After a routine Aortic balloon valvuloplasty, a 22F or 24F sheath is advanced from the femoral artery to the aorta. The manipulation of the prosthesis around the aortic arch and through the stenotic valve is facilitated by a steerable, deflectable catheter. Rapid ventricular pacing is used to decrease cardiac output while the delivery balloon is inflated to deploy the prosthesis within the annulus.
- Transapical antegrade approach[8]: An alternate catheter based method consists of a direct left ventricular apical puncture and antegrade aortic valve implantation via a small anterolateral thoracotomy without the need of cardiopulmonary bypass or sternotomy. This technique is used in patients with severe peripheral arterial disease and heavily calcified ascending aorta and arch (porcelain aorta) who have an increased risk of stroke and other embolic events using other approaches.
Alternative vascular access
In some patients, the peripheral vascular anatomy is unsuitable for a transfemoral approach; for such reason a number of other vascular access have been suggested. The Subclavian (ie,axillary) or Transaortic access may be useful in solving such problems.[9]In a series of 54 cases treated via the Subclavian approach in the Italian National Registry, procedural success was achieved in 100% of cases.[10] No specific complications such as vessel rupture or vertebral or internal mammary ischemia associated with Subclavian access were found. No deaths at 30 days in this series, and the 6-month mortality rate was 9.4% and was no different from those who underwent a transfemoral approach.
Complications
Strokes and Transient Ischemic Attacks
The etiology of cerebrovascular events after TAVI is thought to be related to the embolization of atherothrombotic material during advancement of the device to and across the aortic valve.[11]Magnetic resonnace imaging have shown that microembolization is common with both balloon-expandable and self-expanding percutaneous valves, as well with surgical aortic valve repair (SAVR), but the presence of clinical strokes are infrequent (2.9%-%5.1%)
Aortic Regurgitation
Significant aortic regurgitation caused by paravalvular leak after CoreValve percutaneous implantation is usually an uncommon complication that relates more frequently to low positioning of the CoreValve frame, incomplete expansion of the frame into the eccentrically shaped annulus, rigidity of the underlying aortic annulus due to calcium, or undersizing of the valve relative to the aortic annular size.[12]
Vascular access complications
The relatively large-caliber sheath (18F) required for placement of the percutaneous valve may be the cause of various vascular complications. One of the most common vascular events encountered are incomplete arteriotomy closure.[11]Avoiding such complications is possible; preprocedural screening using computed tomographic angiography, vascular ultrasound guidance for arterial access, and alternative (eg, subclavian) access have allowed better selection to avoid those vascular complications.ref
Coronary Artery Occlusion
Coronary occlusion after TAVI is usually rare but may occur in some cases due to expansion of the native aortic valve across the orifice of the coronary ostium. This could be prevented with careful preprocedural screening to ensure adequate sinus of valsava width (30mm) and height (15mm).[13]
Conduction abnormalities
Worsening or new conduction abnormalities are frequently observed with TAVI; more often when self-expandable CoreValve device is used[14][15]. Conduction abnormalities may be due to compression of superficially running left bundle branch (in the uppermost part of ventricular septum) by the lower one third of prosthesis which exerts radial forces for secure anchoring of the stent against the native annulus and outflow septum. Hence, deeper the implantation of the prosthesis into the left ventricular outflow tract, greater is the risk of development of severe conduction defect requiring pacemaker implantation.
A study in Italy reported that 77% of the patients post TAVI developed new onset or worsening of per-existing conduction abnormalities. 44% of the patients developed left bundle branch block(LBBB) and subsequently 39% of the patients underwent implantation of pacemaker. After TAVI, 6 (75%) of 8 patients with right bundle branch block(RBBB) at baseline required pacemaker implantation versus 19 (34%) of 56 patients, who had not had RBBB before TAVI. It was concluded that the RBBB was the only baseline conduction abnormality that significantly affected the occurrence of pacemaker implantation after TAVI because if patients already have a right bundle branch block, then a procedure-induced left bundle branch block will result in a complete atrioventricular block requiring a pacemaker[16][17].
Other CoreValve implantation complications are [18]:
- QRS duration: In one observational study of 270 patients, the QRS duration increased from 105±23 milliseconds at baseline to 135±29 milliseconds following TAVI. (P<0.01).
- Left Bundle Branch Block (LBBB): The incidence of left bundle-branch block increased from 13% at baseline to 61% following TAVI (P<0.001).
- Permanent pacemaker implantation: Approximately one third of patients will require a permanent pacemaker be implanted by 30 days with a median time to insertion of 4 days (interquartile range, 2.0 to 7.75 days).
- Multivariate predictors of permanent pacemaker implantation included:
- Periprocedural atrioventricular block (odds ratio, 6.29; 95% confidence interval, 3.55 to 11.15)
- Balloon pre-dilatation (odds ratio, 2.68; 95% confidence interval, 2.00 to 3.47)
- Use of a larger 29 mm CoreValve prosthesis (odds ratio, 2.50; 95% confidence interval, 1.22 to 5.11)
- The interventricular septum diameter (odds ratio, 1.18; 95% confidence interval, 1.10 to 3.06)
- A prolonged QRS duration (odds ratio, 3.45; 95% confidence interval, 1.61 to 7.40)
Future Perspectives
TAVI has transformed the treatment and the way of dealing with symptomatic patients suffering from aortic stenosis, particularly in those who are high risk or inoperable for surgical aortic valve replacement (SAVR).[19] The future of TAVI will be focusing on more technical aspects; trying to reduce the device profile, enhancing it's positioning, retrievability and promoting valve durability with anticalcification treatments. With ongoing clinical trials and further evidence that will come up, TAVI will be a valuable treatment alternative for patients with severe Aortic stenosis unqualified for SAVR and will be a life saving procedure to many of those patients.
References
- ↑ 1.0 1.1 Grube E, Laborde JC, Gerckens U, Felderhoff T, Sauren B, Buellesfeld L, Mueller R, Menichelli M, Schmidt T, Zickmann B, Iversen S, Stone GW (2006). "Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study". Circulation. 114 (15): 1616–24. doi:10.1161/CIRCULATIONAHA.106.639450. PMID 17015786. Retrieved 2011-03-17. Unknown parameter
|month=ignored (help) - ↑ Heart Mirror J 2010; 157-159.
- ↑ CARDIAC INTERVENTIONS TODAY,TAVI using the CoreValve revalving system,July.August 2010
- ↑ Heart Mirror J 2010; 157-159.
- ↑ Vavuranakis M, Voudris V, Vrachatis DA, Thomopoulou S, Toutouzas K, Karavolias G, Tolios I, Sbarouni E, Lazaros G, Chrysohoou C, Khoury M, Brili S, Balanika M, Moldovan C, Stefanadis C (2010). "Transcatheter aortic valve implantation, patient selection process and procedure: two centres' experience of the intervention without general anaesthesia" (PDF). Hellenic Journal of Cardiology : HJC = Hellēnikē Kardiologikē Epitheōrēsē. 51 (6): 492–500. PMID 21169181. Retrieved 2011-03-21.
- ↑ Zajarias A, Cribier AG (2009). "Outcomes and safety of percutaneous aortic valve replacement". Journal of the American College of Cardiology. 53 (20): 1829–36. doi:10.1016/j.jacc.2008.11.059. PMID 19442881. Retrieved 2011-03-18. Unknown parameter
|month=ignored (help) - ↑ Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI, Buller CE, Pasupati S, Lichtenstein S (2006). "Percutaneous aortic valve implantation retrograde from the femoral artery". Circulation. 113 (6): 842–50. doi:10.1161/CIRCULATIONAHA.105.582882. PMID 16461813. Retrieved 2011-03-18. Unknown parameter
|month=ignored (help) - ↑ Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H, Eggebrecht H, Rubino P, Michev I, Lange R, Anderson WN, Wendler O (2010). "Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve". Circulation. 122 (1): 62–9. doi:10.1161/CIRCULATIONAHA.109.907402. PMID 20566953. Retrieved 2011-03-18. Unknown parameter
|month=ignored (help) - ↑ Fraccaro C, Napodano M, Tarantini G, Gasparetto V, Gerosa G, Bianco R, Bonato R, Pittarello D, Isabella G, Iliceto S, Ramondo A (2009). "Expanding the eligibility for transcatheter aortic valve implantation the trans-subclavian retrograde approach using: the III generation CoreValve revalving system". JACC. Cardiovascular Interventions. 2 (9): 828–33. doi:10.1016/j.jcin.2009.06.016. PMID 19778770. Retrieved 2011-03-23. Unknown parameter
|month=ignored (help) - ↑ Ruge H, Lange R, Bleiziffer S, Hutter A, Mazzitelli D, Will A, Schreiber C, Laborde JC, Bauernschmitt R (2008). "First successful aortic valve implantation with the CoreValve ReValving System via right subclavian artery access: a case report". The Heart Surgery Forum. 11 (5): E323–4. doi:10.1532/HSF98.20081021. PMID 18948247. Retrieved 2011-03-23.
- ↑ 11.0 11.1 Kahlert P, Knipp SC, Schlamann M, Thielmann M, Al-Rashid F, Weber M, Johansson U, Wendt D, Jakob HG, Forsting M, Sack S, Erbel R, Eggebrecht H (2010). "Silent and apparent cerebral ischemia after percutaneous transfemoral aortic valve implantation: a diffusion-weighted magnetic resonance imaging study". Circulation. 121 (7): 870–8. doi:10.1161/CIRCULATIONAHA.109.855866. PMID 20177005. Retrieved 2011-03-22. Unknown parameter
|month=ignored (help) - ↑ Jilaihawi H, Chin D, Vasa-Nicotera M, Jeilan M, Spyt T, Ng GA, Bence J, Logtens E, Kovac J (2009). "Predictors for permanent pacemaker requirement after transcatheter aortic valve implantation with the CoreValve bioprosthesis". American Heart Journal. 157 (5): 860–6. doi:10.1016/j.ahj.2009.02.016. PMID 19376312. Retrieved 2011-03-22. Unknown parameter
|month=ignored (help) - ↑ Gerckens U, Latsios G, Mueller R, et al. Left main PCI after trans-subclavian CoreValve implantation. Successful outcome of a combined procedure for management of a rare complication.Clin Res Cardiol. 2009;98:687-690.
- ↑ Piazza N, Onuma Y, Jesserun E, Kint PP, Maugenest AM, Anderson RH; et al. (2008). "Early and persistent intraventricular conduction abnormalities and requirements for pacemaking after percutaneous replacement of the aortic valve". JACC Cardiovasc Interv. 1 (3): 310–6. doi:10.1016/j.jcin.2008.04.007. PMID 19463319.
- ↑ Piazza N, Grube E, Gerckens U, den Heijer P, Linke A, Luha O; et al. (2008). "Procedural and 30-day outcomes following transcatheter aortic valve implantation using the third generation (18 Fr) corevalve revalving system: results from the multicentre, expanded evaluation registry 1-year following CE mark approval". EuroIntervention. 4 (2): 242–9. PMID 19110790.
- ↑ Fraccaro C, Buja G, Tarantini G, Gasparetto V, Leoni L, Razzolini R; et al. (2011). "Incidence, predictors, and outcome of conduction disorders after transcatheter self-expandable aortic valve implantation". Am J Cardiol. 107 (5): 747–54. doi:10.1016/j.amjcard.2010.10.054. PMID 21247519.
- ↑ http://www.medpagetoday.com/MeetingCoverage/EUROPACE/27358 accessed on July 1, 2011
- ↑ Khawaja MZ, Rajani R, Cook A, Khavandi A, Moynagh A, Chowdhary S, Spence MS, Brown S, Khan SQ, Walker N, Trivedi U, Hutchinson N, De Belder AJ, Moat N, Blackman DJ, Levy RD, Manoharan G, Roberts D, Khogali SS, Crean P, Brecker SJ, Baumbach A, Mullen M, Laborde JC, Hildick-Smith D (2011). "Permanent Pacemaker Insertion After CoreValve Transcatheter Aortic Valve Implantation: Incidence and Contributing Factors (the UK CoreValve Collaborative)". Circulation. doi:10.1161/CIRCULATIONAHA.109.927152. PMID 21339482. Retrieved 2011-02-23. Unknown parameter
|month=ignored (help) - ↑ CARDIAC INTERVENTIONS TODAY,TAVI using the CoreValve revalving system,July.August 2010