Transcatheter aortic valve replacement
Editors-in-Chief: Roger Laham, M.D., C. Michael Gibson, M.S., M.D.; Jeffrey J. Popma, M.D.
Assistant Editor-In-Chief: Saleh El Dassouki, MD [1]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Synonyms and keywords: TAVI, Edward's valve, Edward's SAPIEN transcatheter heart valve, CoreValve, percutaneous aortic valve replacement, PAVR
Overview
Untill recently, aortic valve replacement (AVR) was the only effective treatment to severe symptomatic aortic stenosis. However, over the past decade percutaneous treatment of aortic valve disease with implantation of a stent-based valve prosthesis has been introduced as a new treatment in patients considered inoperable because of severe co-morbidities.[1] In Transcatheter Aortic Valve Implantation (TAVI) also known as Percutaneous Aortic Valve Replacement (PAVR), a synthetic valve is transported to the heart through a small hole made in groin. This procedure can be compared to that performed when placing a stent, or performing balloon angioplasty. Traditional aortic valve replacement is an invasive surgical procedure, with considerable mortality and morbidity, especially in more fragile patients. In the newly developed TAVI procedure, the dysfunctional aortic valve is replaced percutaneously, which removes the need for open heart surgery.
Valve types
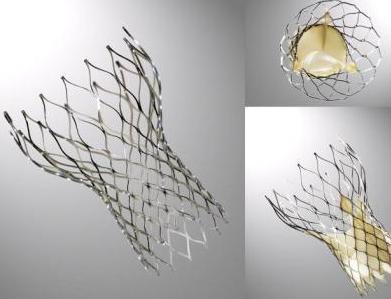
CoreValve
The CoreValve device was first inserted in 2005[1].[2]It consists of three leaflets of bioprosthetic pericardial tissue valve mounted on a self-expendable nitinol stent, which expands from the left ventricular outflow tract (LVOT) to the ascending aorta. Initially the device had a 25F profile, but it rapidly evolved to the current 18F device which lead to easier percutaneous insertion and better handling of the device. A third generation device is currently available, which measures 50 mm in height that is divided into 3 sections:
- An inflow portion designed to fix the valve to the annulus.
- An outflow portion designed to attach the frame of the device to the ascending aorta to stabilize it.
- A central portion designed to prevent the obstruction of the coronaries.
Complications
Following CoreValve implantation[3]:
- QRS duration: In one observational study of 270 patients, the QRS duration increased from 105±23 milliseconds at baseline to 135±29 milliseconds following TAVI. (P<0.01).
- Left Bundle Branch Block (LBBB): The incidence of left bundle-branch block increased from 13% at baseline to 61% following TAVI (P<0.001).
- Permanent pacemaker implantation: Approximately one third of patients will require a permanent pacemaker be implanted by 30 days with a median time to insertion of 4 days (interquartile range, 2.0 to 7.75 days).
- Multivariate predictors of permanent pacemaker implantation included:
- Periprocedural atrioventricular block (odds ratio, 6.29; 95% confidence interval, 3.55 to 11.15)
- Balloon pre-dilatation (odds ratio, 2.68; 95% confidence interval, 2.00 to 3.47)
- Use of a larger 29 mm CoreValve prosthesis (odds ratio, 2.50; 95% confidence interval, 1.22 to 5.11)
- The interventricular septum diameter (odds ratio, 1.18; 95% confidence interval, 1.10 to 3.06)
- A prolonged QRS duration (odds ratio, 3.45; 95% confidence interval, 1.61 to 7.40)
References
- ↑ 1.0 1.1 Grube E, Laborde JC, Gerckens U, Felderhoff T, Sauren B, Buellesfeld L, Mueller R, Menichelli M, Schmidt T, Zickmann B, Iversen S, Stone GW (2006). "Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study". Circulation. 114 (15): 1616–24. doi:10.1161/CIRCULATIONAHA.106.639450. PMID 17015786. Retrieved 2011-03-17. Unknown parameter
|month=ignored (help) - ↑ Heart Mirror J 2010; 157-159.
- ↑ Khawaja MZ, Rajani R, Cook A, Khavandi A, Moynagh A, Chowdhary S, Spence MS, Brown S, Khan SQ, Walker N, Trivedi U, Hutchinson N, De Belder AJ, Moat N, Blackman DJ, Levy RD, Manoharan G, Roberts D, Khogali SS, Crean P, Brecker SJ, Baumbach A, Mullen M, Laborde JC, Hildick-Smith D (2011). "Permanent Pacemaker Insertion After CoreValve Transcatheter Aortic Valve Implantation: Incidence and Contributing Factors (the UK CoreValve Collaborative)". Circulation. doi:10.1161/CIRCULATIONAHA.109.927152. PMID 21339482. Retrieved 2011-02-23. Unknown parameter
|month=ignored (help)