Transcatheter aortic valve replacement: Difference between revisions
No edit summary |
No edit summary |
||
| Line 23: | Line 23: | ||
[[Image:CoreValve-Prosthesis-.jpg|350px]] | [[Image:CoreValve-Prosthesis-.jpg|350px]] | ||
==Procedure== | |||
The diseased valve is moved aside by the inflation of a balloon [[Aortic balloon valvuloplasty]], than the Corevalve prosthesis is loaded in a specialized delivery catheter and advanced to the stenosed aortic valve. Once correctly positioned, the external part of the delivery system is progressively retracted, deploying the Corevalve Prosthesis. The delivery catheter is then closed and retrieved. | |||
===Techniques=== | |||
Two major catheter based techniques for replacing the aortic valve have been investigated <ref name="pmid19442881">{{cite journal |author=Zajarias A, Cribier AG |title=Outcomes and safety of percutaneous aortic valve replacement |journal=[[Journal of the American College of Cardiology]] |volume=53 |issue=20 |pages=1829–36 |year=2009 |month=May |pmid=19442881 |doi=10.1016/j.jacc.2008.11.059 |url=http://linkinghub.elsevier.com/retrieve/pii/S0735-1097(09)00702-5 |accessdate=2011-03-18}}</ref>: | |||
retrograde percutaneous implantation and direct apical puncture. An antegrade transseptal approach has also been studied but not fully adopted. | |||
*Retrograde approach<ref name="pmid16461813">{{cite journal |author=Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI, Buller CE, Pasupati S, Lichtenstein S |title=Percutaneous aortic valve implantation retrograde from the femoral artery |journal=[[Circulation]] |volume=113 |issue=6 |pages=842–50 |year=2006 |month=February |pmid=16461813 |doi=10.1161/CIRCULATIONAHA.105.582882 |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=16461813 |accessdate=2011-03-18}}</ref>: After a routine [[Aortic balloon valvuloplasty]], a 22F or 24F sheath is advanced from the femoral artery to the aorta. The manipulation of the prosthesis around the aortic arch and through the stenotic valve is facilitated by a steerable, deflectable catheter. Rapid ventricular pacing is used to decrease cardiac output while the delivery balloon is inflated to deploy the prosthesis within the annulus. | |||
*Transapical antegrade approach<ref name="pmid20566953">{{cite journal |author=Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H, Eggebrecht H, Rubino P, Michev I, Lange R, Anderson WN, Wendler O |title=Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve |journal=[[Circulation]] |volume=122 |issue=1 |pages=62–9 |year=2010 |month=July |pmid=20566953 |doi=10.1161/CIRCULATIONAHA.109.907402 |url=http://circ.ahajournals.org/cgi/pmidlookup?view=long&pmid=20566953 |accessdate=2011-03-18}}</ref>: An alternate catheter based method consists of a direct left ventricular apical puncture and antegrade aortic valve implantation via a small anterolateral thoracotomy without the need of cardiopulmonary bypass or sternotomy. This technique is used in patients with severe peripheral arterial disease and heavily calcified ascending aorta and arch (porcelain aorta) who have an increased risk of stroke and other embolic events using other approaches. | |||
Revision as of 15:16, 18 March 2011
Editors-in-Chief: Roger Laham, M.D., C. Michael Gibson, M.S., M.D.; Jeffrey J. Popma, M.D.
Assistant Editor-In-Chief: Saleh El Dassouki, MD [1]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [2] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Synonyms and keywords: TAVI, Edward's valve, Edward's SAPIEN transcatheter heart valve, CoreValve, percutaneous aortic valve replacement, PAVR
Overview
Untill recently, aortic valve replacement (AVR) was the only effective treatment to severe symptomatic aortic stenosis. However, over the past decade percutaneous treatment of aortic valve disease with implantation of a stent-based valve prosthesis has been introduced as a new treatment in patients considered inoperable because of severe co-morbidities.[1] In Transcatheter Aortic Valve Implantation (TAVI) also known as Percutaneous Aortic Valve Replacement (PAVR), a synthetic valve is transported to the heart through a small hole made in groin. This procedure can be compared to that performed when placing a stent, or performing balloon angioplasty. Traditional aortic valve replacement is an invasive surgical procedure, with considerable mortality and morbidity, especially in more fragile patients. In the newly developed TAVI procedure, the dysfunctional aortic valve is replaced percutaneously, which removes the need for open heart surgery.
Valve types
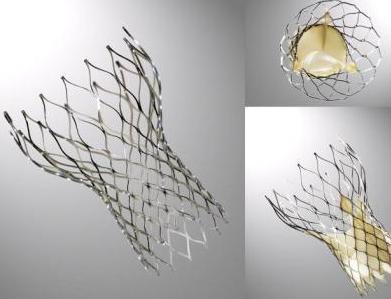
CoreValve
The CoreValve device was first inserted in 2005[1].[2]It consists of three leaflets of bioprosthetic pericardial tissue valve mounted on a self-expendable nitinol stent, which expands from the left ventricular outflow tract (LVOT) to the ascending aorta. Initially the device had a 25F profile, but it rapidly evolved to the current 18F device which lead to easier percutaneous insertion and better handling of the device. A third generation device is currently available, which measures 50 mm in height that is divided into 3 sections:
- An inflow portion designed to fix the valve to the annulus.
- An outflow portion designed to attach the frame of the device to the ascending aorta to stabilize it.
- A central portion designed to prevent the obstruction of the coronaries.
Procedure
The diseased valve is moved aside by the inflation of a balloon Aortic balloon valvuloplasty, than the Corevalve prosthesis is loaded in a specialized delivery catheter and advanced to the stenosed aortic valve. Once correctly positioned, the external part of the delivery system is progressively retracted, deploying the Corevalve Prosthesis. The delivery catheter is then closed and retrieved.
Techniques
Two major catheter based techniques for replacing the aortic valve have been investigated [3]: retrograde percutaneous implantation and direct apical puncture. An antegrade transseptal approach has also been studied but not fully adopted.
- Retrograde approach[4]: After a routine Aortic balloon valvuloplasty, a 22F or 24F sheath is advanced from the femoral artery to the aorta. The manipulation of the prosthesis around the aortic arch and through the stenotic valve is facilitated by a steerable, deflectable catheter. Rapid ventricular pacing is used to decrease cardiac output while the delivery balloon is inflated to deploy the prosthesis within the annulus.
- Transapical antegrade approach[5]: An alternate catheter based method consists of a direct left ventricular apical puncture and antegrade aortic valve implantation via a small anterolateral thoracotomy without the need of cardiopulmonary bypass or sternotomy. This technique is used in patients with severe peripheral arterial disease and heavily calcified ascending aorta and arch (porcelain aorta) who have an increased risk of stroke and other embolic events using other approaches.
Complications
Following CoreValve implantation[6]:
- QRS duration: In one observational study of 270 patients, the QRS duration increased from 105±23 milliseconds at baseline to 135±29 milliseconds following TAVI. (P<0.01).
- Left Bundle Branch Block (LBBB): The incidence of left bundle-branch block increased from 13% at baseline to 61% following TAVI (P<0.001).
- Permanent pacemaker implantation: Approximately one third of patients will require a permanent pacemaker be implanted by 30 days with a median time to insertion of 4 days (interquartile range, 2.0 to 7.75 days).
- Multivariate predictors of permanent pacemaker implantation included:
- Periprocedural atrioventricular block (odds ratio, 6.29; 95% confidence interval, 3.55 to 11.15)
- Balloon pre-dilatation (odds ratio, 2.68; 95% confidence interval, 2.00 to 3.47)
- Use of a larger 29 mm CoreValve prosthesis (odds ratio, 2.50; 95% confidence interval, 1.22 to 5.11)
- The interventricular septum diameter (odds ratio, 1.18; 95% confidence interval, 1.10 to 3.06)
- A prolonged QRS duration (odds ratio, 3.45; 95% confidence interval, 1.61 to 7.40)
References
- ↑ 1.0 1.1 Grube E, Laborde JC, Gerckens U, Felderhoff T, Sauren B, Buellesfeld L, Mueller R, Menichelli M, Schmidt T, Zickmann B, Iversen S, Stone GW (2006). "Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study". Circulation. 114 (15): 1616–24. doi:10.1161/CIRCULATIONAHA.106.639450. PMID 17015786. Retrieved 2011-03-17. Unknown parameter
|month=ignored (help) - ↑ Heart Mirror J 2010; 157-159.
- ↑ Zajarias A, Cribier AG (2009). "Outcomes and safety of percutaneous aortic valve replacement". Journal of the American College of Cardiology. 53 (20): 1829–36. doi:10.1016/j.jacc.2008.11.059. PMID 19442881. Retrieved 2011-03-18. Unknown parameter
|month=ignored (help) - ↑ Webb JG, Chandavimol M, Thompson CR, Ricci DR, Carere RG, Munt BI, Buller CE, Pasupati S, Lichtenstein S (2006). "Percutaneous aortic valve implantation retrograde from the femoral artery". Circulation. 113 (6): 842–50. doi:10.1161/CIRCULATIONAHA.105.582882. PMID 16461813. Retrieved 2011-03-18. Unknown parameter
|month=ignored (help) - ↑ Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H, Eggebrecht H, Rubino P, Michev I, Lange R, Anderson WN, Wendler O (2010). "Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) Registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN valve". Circulation. 122 (1): 62–9. doi:10.1161/CIRCULATIONAHA.109.907402. PMID 20566953. Retrieved 2011-03-18. Unknown parameter
|month=ignored (help) - ↑ Khawaja MZ, Rajani R, Cook A, Khavandi A, Moynagh A, Chowdhary S, Spence MS, Brown S, Khan SQ, Walker N, Trivedi U, Hutchinson N, De Belder AJ, Moat N, Blackman DJ, Levy RD, Manoharan G, Roberts D, Khogali SS, Crean P, Brecker SJ, Baumbach A, Mullen M, Laborde JC, Hildick-Smith D (2011). "Permanent Pacemaker Insertion After CoreValve Transcatheter Aortic Valve Implantation: Incidence and Contributing Factors (the UK CoreValve Collaborative)". Circulation. doi:10.1161/CIRCULATIONAHA.109.927152. PMID 21339482. Retrieved 2011-02-23. Unknown parameter
|month=ignored (help)