Tramadol
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tramadol is a opioid analgesic that is FDA approved for the treatment of management of moderate to moderately severe chronic pain in adults who require around-the-clock treatment of their pain for an extended period of time. Common adverse reactions include seizure risk, suicide risk, serotonin syndrome, anaphylactoid and allergic reactions, respiratory depression, withdrawal symptoms.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- It is indicated for the management of moderate to moderately severe chronic pain in adults who require around-the-clock treatment of their pain for an extended period of time.
- 100 mg Capsules: White capsule imprinted with blue ink “G 252” on cap and “100” between lines on the body
- 200 mg Capsules: White capsule imprinted with violet ink “G 253” on cap and “200” between lines on the body
- 300 mg Capsules: White capsule imprinted with red ink “G 254” on cap and “300” between lines on the body
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Tramadol in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tramadol in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Tramadol in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Tramadol in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Tramadol in pediatric patients.
Contraindications
- Tramadol is contraindicated in patients who have previously demonstrated hypersensitivity to tramadol, any other component of ConZip™, or opioids. Reactions range from pruritis to fatal anaphylactoid reactions.
- ConZip™ is contraindicated in patients with significant respiratory depression in unmonitored settings or the absence of resuscitative equipment.
- ConZip™ is contraindicated in patients with acute or severe bronchial asthma or hypercapnia in unmonitored settings or the absence of resuscitative equipment.
Warnings
Seizure Risk
- Seizures have been reported in patients receiving tramadol within the recommended dosage range. Spontaneous post-marketing reports indicate that seizure risk is increased with doses of tramadol above the recommended range. * Concomitant use of tramadol increases the seizure risk in patients taking:
- Selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors (SNRIs) antidepressants or anorectics,
Tricyclic antidepressants (TCAs), and other tricyclic compounds (e.g., cyclobenzaprine, promethazine, etc.), other opioids, MAO inhibitors, neuroleptics, or other drugs that reduce the seizure threshold.
- Risk of seizures may also increase in patients with epilepsy, those with a history of seizures, or in patients with a recognized risk for seizure (such as head trauma, metabolic disorders, alcohol and drug withdrawal, CNS infections).
- In tramadol overdose, naloxone administration may increase the risk of seizure.
Suicide Risk
- Do not prescribe ConZip™ for patients who are suicidal or addiction-prone. Consideration should be given to the use of non-narcotic analgesics in patients who are suicidal or depressed.
- Prescribe ConZip™ with caution for patients with a history of misuse and/or are taking CNS-active drugs including tranquilizers or antidepressant drugs, or alcohol in excess, and patients who suffer from emotional disturbance or depression.
- Tell your patients not to exceed the recommended dose and to limit their intake of alcohol.
Serotonin Syndrome Risk
- The development of a potentially life-threatening serotonin syndrome may occur with use of tramadol products, including ConZip™ , particularly with concomitant use of serotonergic drugs such as SSRIs, SNRIs, TCAs, MAOIs and triptans, with drugs which impair metabolism of serotonin (including MAOIs) and with drugs which impair metabolism of tramadol (CYP2D6 and CYP3A4 inhibitors).
- This may occur within the recommended dose.
- Serotonin syndrome may include mental-status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
Anaphylactoid Reactions
- Serious and rarely fatal anaphylactoid reactions have been reported in patients receiving therapy with tramadol. When these events do occur it is often following the first dose.
- Other reported allergic reactions include pruritus, hives, bronchospasm, angioedema, toxic epidermal necrolysis and Stevens-Johnson syndrome. Patients with a history of anaphylactoid reactions to codeine and other opioids may be at increased risk and therefore should not receive ConZip™.
Respiratory Depression
- Administer ConZip™ cautiously in patients at risk for respiratory depression.
- In these patients alternative non-opioid analgesics should be considered. If large doses of tramadol are administered with anesthetic medications or alcohol, respiratory depression may result. Respiratory depression should be treated as an overdose.
- If naloxone is to be administered, use cautiously because it may precipitate seizures.
Interaction With Central Nervous System (CNS) Depressants, Including Alcohol and Drugs of Abuse
- Tramadol may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression. Use ConZip™ with caution and in reduced dosages when administered to patients receiving CNS depressants such as alcohol, opioids, anesthetic agents, narcotics, phenothiazines, tranquilizers or sedative hypnotics.
- ConZip™ increases the risk of CNS and respiratory depression in these patients. Alcohol-containing beverages should not be consumed by patients using ConZip™.
Patients with Increased Intracranial Pressure or Head Trauma
- Use ConZip™ with caution in patients with increased intracranial pressure or head injury.
- The respiratory depressant effects of opioids include carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure, and may be markedly exaggerated in these patients.
- Additionally, pupillary changes (miosis) from tramadol may obscure the existence, extent, or course of intracranial pathology. Clinicians should also maintain a high index of suspicion for adverse drug reaction when evaluating altered mental status in these patients if they are receiving ConZip™.
Use in Ambulatory Patients
- ConZip™ may impair the mental and or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
- Caution patients initiating therapy with ConZip™ or those whose dose has been increased to refrain from potentially hazardous activities until it is established that their mental and physical abilities are not significantly impaired.
Use With MAO Inhibitors and SSRIs
- Use ConZip™ with great caution in patients taking monoamine oxidase inhibitors.
- Animal studies have shown increased deaths with combined administration. Concomitant use of ConZip™ with MAO inhibitors or SSRI's increases the risk of adverse reactions, including seizure and serotonin syndrome.
Withdrawal Symptoms
- Withdrawal symptoms may occur if ConZip™ is discontinued abruptly. These symptoms may include: anxiety, sweating, insomnia, rigors, pain, nausea, tremors, diarrhea, upper respiratory symptoms, piloerection, and rarely hallucinations.
- Clinical experience with other formulations of tramadol suggests that withdrawal symptoms may be reduced by tapering ConZip™ when discontinuing tramadol therapy.
Misuse, Abuse and Diversion of Opioids
- ConZip™ contains tramadol, an opioid agonist of the morphine-type. Such drugs are sought by drug abusers and people with addiction disorders and are subject to criminal diversion.
- Tramadol can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing ConZip™ in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse, or diversion.
- ConZip™ could be abused by crushing, chewing, snorting, or injecting the dissolved product. These practices will result in the uncontrolled delivery of the opioid and pose a significant risk to the abuser that could result in overdose and death.
- Concerns about abuse, addiction, and diversion should not prevent the proper management of pain. The development of addiction to opioid analgesics in properly managed patients with pain has been reported to be rare. However, data are not available to establish the true incidence of addiction in chronic pain patients.
- Healthcare professionals should contact their State Professional Licensing Board, or State Controlled Substances Authority for information on how to prevent and detect abuse or diversion of this product.
Risk of Overdosage
- Serious potential consequences of overdosage with ConZip™ are central nervous system depression, respiratory depression and death. In treating an overdose, primary attention should be given to maintaining adequate ventilation along with general supportive treatment.
Acute Abdominal Conditions
- The administration of ConZip™ may complicate the clinical assessment of patients with acute abdominal conditions.
Adverse Reactions
Clinical Trials Experience
- The serious or otherwise important adverse reactions are following.
- Seizure risk
- Suicide risk
- Serotonin syndrome
- Anaphylactoid and allergic reactions
- Respiratory depression
- Withdrawal symptoms
Clinical Studies Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
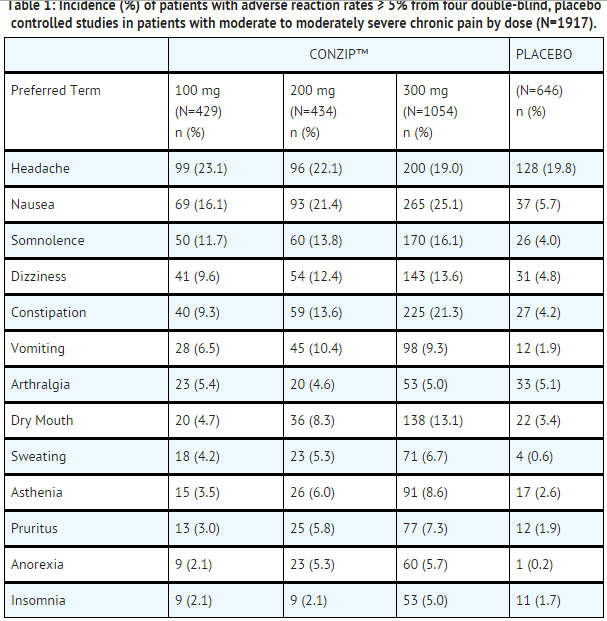
- ConZip™ capsules were administered to a total of 1987 patients in clinical trials. These included four double-blind and one long-term, open-label study in patients with osteoarthritis of the hip and knee. A total of 812 patients were 65 years or older.
- Adverse reactions with doses from 100 mg to 300 mg in the four pooled, randomized, double-blind, placebo-controlled studies in patients with chronic non-malignant pain are presented in the following table.
Adverse Reactions with Incidence Rates of 1.0% to <5.0%
- Cardiac disorders: hypertension
- Gastrointestinal disorders: dyspepsia, flatulence
- General disorders: abdominal pain, accidental injury, chills, fever, flu syndrome, neck pain, pelvic pain
- Investigations: hyperglycemia, urine abnormality
Metabolism and nutrition disorders: peripheral edema, weight loss
- Musculoskeletal, connective tissue and bone disorders: myalgia
- Nervous system disorders: paresthesia, tremor, withdrawal syndrome
- Psychiatric disorders: agitation, anxiety, apathy, confusion, depersonalization, depression, euphoria, nervousness
- Respiratory, thoracic and mediastinal disorders: bronchitis, pharyngitis, rhinitis, sinusitis
- Skin and subcutaneous tissue disorders: rash
- Urogenital disorders: prostatic disorder, urinary tract infection
- Vascular disorders: vasodilatation
Adverse Reactions with Incidence Rates of 0.5% to <1.0% at Any Dose and Serious Adverse Reactions Reported in at least Two Patients
- Cardiac disorders: EKG abnormal, hypotension, tachycardia
- Gastrointestinal disorders: gastroenteritis
- General disorders: neck rigidity, viral infection
- Hematologic/Lymphatic disorders; anemia, ecchymoses
- Metabolism and nutrition disorders: blood urea nitrogen increased, GGT increased, gout, SGPT increased
- Musculoskeletal disorders: arthritis, arthrosis, joint disorder, leg cramps
- Nervous system disorders: emotional lability, hyperkinesia, hypertonia, thinking abnormal, twitching, vertigo
- Respiratory disorders: pneumonia
- Skin and subcutaneous tissue disorders: hair disorder, skin disorder, urticaria
- Special Senses: eye disorder, lacrimation disorder
- Urogenital disorders: cystitis, dysuria, sexual function abnormality, urinary retention
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Tramadol in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tramadol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tramadol during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Tramadol with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Tramadol with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Tramadol with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Tramadol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tramadol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Tramadol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Tramadol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tramadol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tramadol in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Tramadol in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Tramadol in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Tramadol in the drug label.
Pharmacology
There is limited information regarding Tramadol Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Tramadol in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Tramadol in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Tramadol in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Tramadol in the drug label.
How Supplied
Storage
There is limited information regarding Tramadol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Tramadol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Tramadol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Tramadol in the drug label.
Precautions with Alcohol
- Alcohol-Tramadol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Tramadol
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Tramadol |Label Name=Tramadol11.png
}}
{{#subobject:
|Label Page=Tramadol |Label Name=Tramadol11.png
}}