Toxoplasmosis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 39: | Line 39: | ||
In NHANES 1999–2000, the ''T. gondii'' antibody prevalence was higher among non-Hispanic black persons than non-Hispanic white persons. This finding may reflect immigration patterns from countries with higher rates of ''T. gondii'' infection or soil exposure and culinary practices among these different populations. The seroprevalence among persons born outside the United States tended to be higher in NHANES 1999–2000 than in NHANES III, and the percentage of persons born outside the United States tended to be higher in NHANES 1999–2000 than NHANES III, but these findings were not statistically significant. Clearly, in both NHANES III and NHANES 1999–2000 the seroprevalence is higher among persons not born in the United States than in U.S.-born persons. The NHANES 1999–2000 sample population is not large enough to stratify racial/ethnic groups by foreign-birth status and obtain accurate estimates; however, in a multivariate analysis reported previously that used NHANES III data, being born outside the United States was a significant risk factor for ''T. gondii'' seropositivity. However, race/ethnicity did not increase risk (using white non-Hispanic persons as the reference group). | In NHANES 1999–2000, the ''T. gondii'' antibody prevalence was higher among non-Hispanic black persons than non-Hispanic white persons. This finding may reflect immigration patterns from countries with higher rates of ''T. gondii'' infection or soil exposure and culinary practices among these different populations. The seroprevalence among persons born outside the United States tended to be higher in NHANES 1999–2000 than in NHANES III, and the percentage of persons born outside the United States tended to be higher in NHANES 1999–2000 than NHANES III, but these findings were not statistically significant. Clearly, in both NHANES III and NHANES 1999–2000 the seroprevalence is higher among persons not born in the United States than in U.S.-born persons. The NHANES 1999–2000 sample population is not large enough to stratify racial/ethnic groups by foreign-birth status and obtain accurate estimates; however, in a multivariate analysis reported previously that used NHANES III data, being born outside the United States was a significant risk factor for ''T. gondii'' seropositivity. However, race/ethnicity did not increase risk (using white non-Hispanic persons as the reference group). | ||
NHANES gives representative estimates of prevalence for the U.S. population but is not designed to evaluate local ''T. gondii'' prevalence levels. Studies have indicated that ''T. gondii'' prevalence varies greatly in the United States; this local variation is most likely related to culinary practices, the ability of oocysts to survive in different climates, and the levels of immigration from areas of the world in which ''T. gondii'' infection is highly endemic. Nevertheless, NHANES produces useful surveillance data for tracking ''T. gondii'' prevalence over time in the United States. We will continue to monitor trends in this nationally representative survey.<ref>http://www.dpd.cdc.gov/dpdx/HTML/Toxoplasmosis.htm</ref><ref> | NHANES gives representative estimates of prevalence for the U.S. population but is not designed to evaluate local ''T. gondii'' prevalence levels. Studies have indicated that ''T. gondii'' prevalence varies greatly in the United States; this local variation is most likely related to culinary practices, the ability of oocysts to survive in different climates, and the levels of immigration from areas of the world in which ''T. gondii'' infection is highly endemic. Nevertheless, NHANES produces useful surveillance data for tracking ''T. gondii'' prevalence over time in the United States. We will continue to monitor trends in this nationally representative survey.<ref>http://www.dpd.cdc.gov/dpdx/HTML/Toxoplasmosis.htm</ref> <ref>http://www.cdc.gov/ncidod/EID/vol9no11/03-0098.htm</ref> | ||
http://www.cdc.gov/ncidod/EID/vol9no11/03-0098.htm | |||
</ref> | |||
The U.S. NHANES (2004-2005) national probability sample found that 33.1% of U.S. persons above 12 years of age had Toxoplasma-specific [[IgG]] antibodies, indicating that they had been infected with the organism. This prevalence has significantly increased from the 1999-2000 data.<ref name=Jones_2003>{{cite journal |author=Jones J, Kruszon-Moran D, Wilson M |title=Toxoplasma gondii infection in the United States, 1999-2000 |journal=Emerg Infect Dis |volume=9 |issue=11 |pages=1371-4 |year=2003 |url=http://www.cdc.gov/ncidod/EID/vol9no11/03-0098.htm |pmid=14718078}}</ref> | The U.S. NHANES (2004-2005) national probability sample found that 33.1% of U.S. persons above 12 years of age had Toxoplasma-specific [[IgG]] antibodies, indicating that they had been infected with the organism. This prevalence has significantly increased from the 1999-2000 data.<ref name=Jones_2003>{{cite journal |author=Jones J, Kruszon-Moran D, Wilson M |title=Toxoplasma gondii infection in the United States, 1999-2000 |journal=Emerg Infect Dis |volume=9 |issue=11 |pages=1371-4 |year=2003 |url=http://www.cdc.gov/ncidod/EID/vol9no11/03-0098.htm |pmid=14718078}}</ref> | ||
It is estimated that between 30% and 65% of all people worldwide are infected with Toxoplasmosis. However, there is large variation countries: in France, for example, around 88% of the population are carriers, probably due to a high consumption of raw and lightly cooked meat. | It is estimated that between 30% and 65% of all people worldwide are infected with Toxoplasmosis. However, there is large variation countries: in France, for example, around 88% of the population are carriers, probably due to a high consumption of raw and lightly cooked meat.<ref>David Adam, Guardian Unlimited. [http://www.guardian.co.uk/life/thisweek/story/0,12977,1048642,00.html ''Can a parasite carried by cats change your personality?''], 25 Sep. 2003</ref> | ||
<ref>David Adam, Guardian Unlimited. [http://www.guardian.co.uk/life/thisweek/story/0,12977,1048642,00.html ''Can a parasite carried by cats change your personality?''], 25 Sep. 2003 | Germany, the Netherlands and Brazil also have high prevalences of around 80%, over 80%<ref>Toxoplasmosis in the Netherlands by the Laboratory for Diagnoses for Infectious Diseases and Screening; RIVM Bilthoven | ||
</ref> | [http://www.nvkc.nl/tijdschrift/content/1999/nr%201/p65/1999-1-p65.pdf]</ref> and 67% respecti ely. In Britain, about 22% are carriers, and South Korea's rate is only 4.3%.<ref name="Zimmer1Aug2006" /> | ||
Germany, the Netherlands and Brazil also have high prevalences of around 80%, over 80% | |||
<ref>Toxoplasmosis in the Netherlands by the Laboratory for Diagnoses for Infectious Diseases and Screening; RIVM Bilthoven | |||
[http://www.nvkc.nl/tijdschrift/content/1999/nr%201/p65/1999-1-p65.pdf]</ref> and 67% | |||
== Risk Factors == | == Risk Factors == | ||
| Line 74: | Line 68: | ||
==== Transmission ==== | ==== Transmission ==== | ||
[[Image:Toxoplasmosis LifeCycle.jpg| | [[Image:Toxoplasmosis LifeCycle.jpg|left|thumb|400px|Life cycle of ''Toxoplasma gondii''.]]Transmission may occur through: | ||
* Ingestion of raw or partly cooked meat, especially pork, lamb, or venison containing Toxoplasma cysts. Infection prevalence in countries where undercooked meat is traditionally eaten, such as France, has been related to this transmission method. Oocysts may also be ingested during hand-to-mouth contact after handling undercooked meat, or from using knives, utensils, or cutting boards contaminated by raw meat.<ref name=CDC>{{cite web | * Ingestion of raw or partly cooked meat, especially pork, lamb, or venison containing Toxoplasma cysts. Infection prevalence in countries where undercooked meat is traditionally eaten, such as France, has been related to this transmission method. Oocysts may also be ingested during hand-to-mouth contact after handling undercooked meat, or from using knives, utensils, or cutting boards contaminated by raw meat.<ref name=CDC>{{cite web | ||
Revision as of 00:27, 18 January 2009
Template:DiseaseDisorder infobox
|
WikiDoc Resources for Toxoplasmosis |
|
Articles |
|---|
|
Most recent articles on Toxoplasmosis Most cited articles on Toxoplasmosis |
|
Media |
|
Powerpoint slides on Toxoplasmosis |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Toxoplasmosis at Clinical Trials.gov Trial results on Toxoplasmosis Clinical Trials on Toxoplasmosis at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Toxoplasmosis NICE Guidance on Toxoplasmosis
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Toxoplasmosis Discussion groups on Toxoplasmosis Patient Handouts on Toxoplasmosis Directions to Hospitals Treating Toxoplasmosis Risk calculators and risk factors for Toxoplasmosis
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Toxoplasmosis |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [4]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [5] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Toxoplasmosis is a parasitic disease caused by the protozoan Toxoplasma gondii.[1] The parasite infects most warm-blooded animals, including humans, but the primary host is the felid (cat) family. Animals are infected by eating infected meat, by ingestion of faeces of a cat that has itself recently been infected, or by transmission from mother to fetus. Cats have been shown as a major reservoir of this infection. [2] While this is true, contact with infected undercooked meat seems to be a more important cause of human infection in many countries.
Up to one third of the world's population is estimated to carry a Toxoplasma infection.[3] The Centers for Disease Control and Prevention notes that overall seroprevalence in the United States as determined with specimens collected by the third National Health and Nutritional Assessment Survey (NHANES III) between 1988 and 1994 was found to be 22.5%, with seroprevalence among women of childbearing age (15 to 44 years) of 15%.[4]
During the first few weeks, the infection typically causes a mild flu-like illness or no illness. After the first few weeks of infection have passed, the parasite rarely causes any symptoms in otherwise healthy adults. However, people with a weakened immune system, such as those infected with HIV, and fetuses, may become seriously ill, and it can occasionally be fatal. The parasite can cause encephalitis (inflammation of the brain) and neurologic diseases and can affect the heart, liver, and eyes (chorioretinitis).
The protozoan was first discovered by Nicolle & Manceaux, who in 1908 isolated it from the African rodent Ctenodactylus gundi, then in 1909 differentiated the disease from Leishmania and named it Toxoplasmosis gondii [5][6]. The first recorded congenital case was not until 1923, and the first adult case not until 1940[5]. In 1948, a serological dye test was created by Sabin & Feldman, which is now the standard basis for diagnostic tests.[6]
Also know as: Toxoplasma Infection
Epidemiology and Demographics
Serologic prevalence data indicate that toxoplasmosis is one of the most common of humans infections throughout the world. Infection is more common in warm climates and at lower altitudes than in cold climates and mountainous regions. High prevalence of infection in France has been related to a preference for eating raw or undercooked meat, while high prevalence in Central America has been related to the frequency of stray cats in a climate favoring survival of oocysts. The overall seroprevalence in the United States as determined with specimens collected by the third National Health and Nutritional Assessment Survey (NHANES III) between 1988 and 1994 was found to be 22.5%, with seroprevalence among women of childbearing age (15 to 44 years) of 15%.
Toxoplasma gondii Infection in the United States, 1999–2000
There was found to be an overall T. gondii IgG antibody prevalence of 15.8% among persons 12–49 years of age in 1999–2000, indicating that approximately 1 in 6 persons in this age group was infected with T. gondii. No significant changes in T. gondii seroprevalence occurred between 1988–1994 and 1999–2000 for the U.S. population as a whole or for any of the subgroups we examined. It was speculated that changes in meat production with lower levels of T. gondii in meat might result in a reduction in the prevalence of T. gondii infection in the population. Perhaps the time was not sufficient for changes in meat production and consumption habits to have had an impact, or perhaps the expected declines in T. gondii infection occurred before NHANES III. The prevalence of T. gondii infection declined in U.S. military recruits, when 1965 data were compared with 1989 data and in countries in Europe, such as France and Belgium, during similar periods.
Predicting future trends in T. gondii prevalence in the United States is difficult because we do not have a national estimate of what proportion of T. gondii infections are attributable to undercooked meat exposure or to cat feces, soil, or water exposure. A large European case-control study that examined these factors showed that undercooked meat accounted for the largest portion of risk for T. gondii infection (30%–63%, depending on location). However, until researchers examine the risk factors for T. gondii infection in a case-control study throughout the United States, the most important U.S. risk factors and how to best focus preventive education messages will not be determined.
In NHANES 1999–2000, the T. gondii antibody prevalence was higher among non-Hispanic black persons than non-Hispanic white persons. This finding may reflect immigration patterns from countries with higher rates of T. gondii infection or soil exposure and culinary practices among these different populations. The seroprevalence among persons born outside the United States tended to be higher in NHANES 1999–2000 than in NHANES III, and the percentage of persons born outside the United States tended to be higher in NHANES 1999–2000 than NHANES III, but these findings were not statistically significant. Clearly, in both NHANES III and NHANES 1999–2000 the seroprevalence is higher among persons not born in the United States than in U.S.-born persons. The NHANES 1999–2000 sample population is not large enough to stratify racial/ethnic groups by foreign-birth status and obtain accurate estimates; however, in a multivariate analysis reported previously that used NHANES III data, being born outside the United States was a significant risk factor for T. gondii seropositivity. However, race/ethnicity did not increase risk (using white non-Hispanic persons as the reference group).
NHANES gives representative estimates of prevalence for the U.S. population but is not designed to evaluate local T. gondii prevalence levels. Studies have indicated that T. gondii prevalence varies greatly in the United States; this local variation is most likely related to culinary practices, the ability of oocysts to survive in different climates, and the levels of immigration from areas of the world in which T. gondii infection is highly endemic. Nevertheless, NHANES produces useful surveillance data for tracking T. gondii prevalence over time in the United States. We will continue to monitor trends in this nationally representative survey.[7] [8]
The U.S. NHANES (2004-2005) national probability sample found that 33.1% of U.S. persons above 12 years of age had Toxoplasma-specific IgG antibodies, indicating that they had been infected with the organism. This prevalence has significantly increased from the 1999-2000 data.[9]
It is estimated that between 30% and 65% of all people worldwide are infected with Toxoplasmosis. However, there is large variation countries: in France, for example, around 88% of the population are carriers, probably due to a high consumption of raw and lightly cooked meat.[10] Germany, the Netherlands and Brazil also have high prevalences of around 80%, over 80%[11] and 67% respecti ely. In Britain, about 22% are carriers, and South Korea's rate is only 4.3%.[12]
Risk Factors
People who are most likely to develop severe toxoplasmosis include:
- Infants born to mothers who became infected with Toxoplasma for the first time during or just before pregnancy.
- Persons with severely weakened immune systems, such as individuals with HIV/AIDS, those taking certain types of chemotherapy, and those who have recently received an organ transplant.
If I am at risk, would I be able to keep my cat?
Yes, you may keep your cat if you are a person at risk for a severe infection (e.g., you have a weakened immune system or are pregnant); however, there are several safety precautions to avoid being exposed to Toxoplasma:
- Keep your cat healthy and help prevent it from becoming infected with Toxoplasma. Keep your cat indoors and feed it dry or canned cat food rather than allowing it to have access to wild birds and rodents or to food scraps. A cat can become infected by eating infected prey or by eating raw or undercooked meat infected with the parasite. Do not bring a new cat into your house that might have spent time out of doors or might have been fed raw meat. Avoid stray cats and kittens and the area they have adopted as their "home." Your veterinarian can answer any other questions you may have regarding your cat and risk for toxoplasmosis.
- Have someone who is healthy and not pregnant change your cat's litter box daily. If this is not possible, wear gloves and clean the litter box every day, because the parasite found in cat feces needs one or more days after being passed to become infectious. Wash your hands well with soap and water afterwards.[13]
Pathophysiology & Etiology
Toxoplasma gondii is a protozoan parasite that infects most species of warm blooded animals, including humans, causing the disease toxoplasmosis.
Members of the cat family (Felidae) are the only known definitive hosts for the sexual stages of T. gondii and thus are the main reservoirs of infection. Cats become infected with T. gondii by carnivorism 1. After tissue cysts or oocysts are ingested by the cat, viable organisms are released and invade epithelial cells of the small intestine where they undergo an asexual followed by a sexual cycle and then form oocysts, which are excreted. The unsporulated oocyst takes 1 to 5 days after excretion to sporulate (become infective). Although cats shed oocysts for only 1 to 2 weeks, large numbers may be shed. Oocysts can survive in the environment for several months and are remarkably resistant to disinfectants, freezing, and drying, but are killed by heating to 70°C for 10 minutes.[14]
Transmission
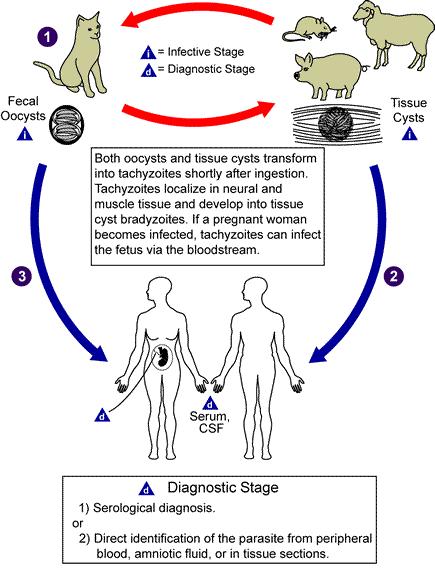
Transmission may occur through:
- Ingestion of raw or partly cooked meat, especially pork, lamb, or venison containing Toxoplasma cysts. Infection prevalence in countries where undercooked meat is traditionally eaten, such as France, has been related to this transmission method. Oocysts may also be ingested during hand-to-mouth contact after handling undercooked meat, or from using knives, utensils, or cutting boards contaminated by raw meat.[15]
- Ingestion of contaminated cat faeces. This can occur through hand-to-mouth contact following gardening, cleaning a cat's litter box, contact with children's sandpits, or touching anything that has come into contact with cat faeces.
- Drinking water contaminated with Toxoplasma.
- Transplacental infection in utero.
- Receiving an infected organ transplant or blood transfusion, although this is extremely rare.[15]
- Accidental inoculation of tachyzoites
Transplacental transmission may occur during different stages:
- infection in 1st trimester - incidence of transplacental infection is low (15%) but disease in neonate is most severe.
- Infection in 3rd trimester - incidence of transplacental infection is high (65%) but infant is usually asymptomatic at birth.
The cyst form of the parasite is extremely hardy, capable of surviving exposure to freezing down to −12 degrees Celsius, moderate temperatures and chemical disinfectants such as bleach, and can survive in the environment for over a year. It is, however, susceptible to high temperatures—above 66 degrees Celsius, and is thus killed by thorough cooking, and would be killed by 24 hours in a typical domestic freezer.[16]
Cats excrete the pathogen in their faeces for a number of weeks after contracting the disease, generally by eating an infected rodent. Even then, cat faeces are not generally contagious for the first day or two after excretion, after which the cyst 'ripens' and becomes potentially pathogenic. Studies have shown that only about 2% of cats are shedding oocysts at any one time, and that oocyst shedding does not recur even after repeated exposure to the parasite. Although the pathogen has been detected on the fur of cats, it has not been found in an infectious form, and direct infection from handling cats is generally believed to be very rare.
Once infected with Toxoplasma is my cat always able to spread the infection to me?
No, cats only spread Toxoplasma in their feces for a few weeks following infection with the parasite. Like humans, cats rarely have symptoms when first infected, so most people do not know if their cat has been infected. The infection will go away on its own; therefore it does not help to have your cat or your cat's feces tested for Toxoplasma.
Diagnosis
The diagnosis of toxoplasmosis may be documented by:
- Observation of parasites in patient specimens, such as bronchoalveolar lavage material from immunocompromised patients, or lymph node biopsy.
- Isolation of parasites from blood or other body fluids, by intraperitoneal inoculation into mice or tissue culture. The mice should be tested for the presence of Toxoplasma organisms in the peritoneal fluid 6 to 10 days post inoculation; if no organisms are found, serology can be performed on the animals 4 to 6 weeks post inoculation.
- Detection of parasite genetic material by PCR, especially in detecting congenital infections in utero.
- Serologic testing is the routine method of diagnosis, because the techniques described above are technically complex and generally not rewarding.
There is an algorithm for the immunodiagnosis of toxoplasmosis for individuals greater than one year of age. The IFA and EIA tests for IgG and IgM antibodies are the tests most commonly used today. Persons should be initially tested for the presence of Toxoplasma-specific IgG antibodies to determine their immune status. A positive IgG titer indicates infection with the organism at some time. If more precise knowledge of the time of infection is necessary, then an IgG positive person should have an IgM test performed by a procedure with minimal nonspecific reactions, such as IgM-capture EIA. A negative IgM test essentially excludes recent infection, but a positive IgM test is difficult to interpret because Toxoplasma-specific IgM antibodies may be detected by EIA for as long as 18 months after acute acquired infection.
A major problem with Toxoplasma-specific IgM testing is lack of specificity. Two situations occur frequently: i) persons with a positive IgM but negative IgG, and ii) individuals with positive IgG and IgM results. In the first situation, a positive IgM result with a negative IgG result in the same specimen should be viewed with great suspicion; the patient's blood should be redrawn two weeks after the first and tested together with the first specimen. If the first specimen was drawn very early after infection, the patient should have highly positive IgG and IgM antibodies in the second sample. If the IgG is negative and the IgM is positive in both specimens, the IgM result should be considered to be a false positive and the patient should be considered to be not infected. In the second situation, a second specimen should be drawn and both specimens submitted together to a reference lab which employs a different IgM testing system for confirmation. Prior to initiation of patient management for acute toxoplasmosis, all IgG/IgM positives should be submitted to a reference lab for IgG avidity testing.
If the patient is pregnant, and IgG/IgM positive, an IgG avidity test should be performed. A high avidity result in the first 12 to 16 weeks of pregnancy (time dependent upon the commercial test kit) essentially rules out an infection acquired during gestation. A low IgG avidity result should not be interpreted as indicating recent infection, because some individuals have persistent low IgG avidity for many months after infection. Suspected recent infection in a pregnant woman should be confirmed prior to intervention by having samples tested at a toxoplasmosis reference laboratory. If the patient has clinical illness compatible with toxoplasmosis but the IgG titer is low, a follow-up titer two to three weeks later should show an increase in antibody titer if the illness is due to acute toxoplasmosis, assuming the host is not severely immunocompromised.
Symptoms
Acquired infection with Toxoplasma in immunocompetent persons is generally an asymptomatic infection. However, 10% to 20% of patients with acute infection may develop cervical lymphadenopathy and/or a flu-like illness. The clinical course is usually benign and self-limited; symptoms usually resolve within a few months to a year. Immunodeficient patients often have central nervous system (CNS) disease but may have retinochoroiditis, or pneumonitis. In patients with AIDS, toxoplasmic encephalitis is the most common cause of intracerebral mass lesions and is thought to be caused by reactivation of chronic infection. Toxoplasmosis in patients being treated with immunosuppressive drugs may be due to either newly acquired or reactivated latent infection.
Acute toxoplasmosis
During acute toxoplasmosis, symptoms are often influenza-like: swollen lymph nodes, or muscle aches and pains that last for a month or more. Rarely, a patient with a fully functioning immune system may develop eye damage from toxoplasmosis. Young children and immunocompromised patients, such as those with HIV/AIDS, those taking certain types of chemotherapy, or those who have recently received an organ transplant, may develop severe toxoplasmosis. This can cause damage to the brain or the eyes. Only a small percentage of infected newborn babies have serious eye or brain damage at birth.
Latent toxoplasmosis
Most patients who become infected with Toxoplasma gondii and develop toxoplasmosis do not know it. In most immunocompetent patients, the infection enters a latent phase, during which only bradyzoites are present, forming cysts in nervous and muscle tissue. Most infants who are infected while in the womb have no symptoms at birth but may develop symptoms later in life.[7]
Congenital toxoplasmosis
Congenital toxoplasmosis results from an acute primary infection acquired by the mother during pregnancy. The incidence and severity of congenital toxoplasmosis vary with the trimester during which infection was acquired. Because treatment of the mother may reduce the incidence of congenital infection and reduce sequelae in the infant, prompt and accurate diagnosis is important. Most infants with subclinical infection at birth will subsequently develop signs or symptoms of congenital toxoplasmosis unless the infection is treated. Ocular Toxoplasma infection, an important cause of retinochoroiditis in the United States, can be the result of congenital infection, or infection after birth. In congenital infection, patients are often asymptomatic until the second or third decade of life, when lesions develop in the eye.[17]
Biological modifications of the host
The parasite itself can cause various effects on the host body, some of which are not fully understood.
Reproductive changes
A recent study [18] has indicated Toxoplasmosis correlates strongly with an increase in boy births in humans. According to the researchers, depending on the antibody concentration, the probability of the birth of a boy can increase up to a value of 0.72 ... which means that for every 260 boys born, 100 girls are born. The study also notes a mean rate of 0.60 to 0.65 (as opposed to the normal 0.51) for Toxoplasma positive mothers.
Behavioral changes
It has been found that the parasite has the ability to change the behavior of its host: infected rats and mice are less fearful of cats — in fact, some of the infected rats seek out cat-urine-marked areas. This effect is advantageous to the parasite, which will be able to sexually reproduce if its host is eaten by a cat.[19] The mechanism for this change is not completely understood, but there is evidence that toxoplasmosis infection raises dopamine levels in infected mice.
The findings of behavioral alteration in rats and mice have led some scientists to speculate that toxoplasma may have similar effects in humans, even in the latent phase that had previously been considered asymptomatic. Toxoplasma is one of a number of parasites that may alter their host's behaviour as a part of their life cycle. [20] The behaviors observed, if caused by the parasite, are likely due to infection and low-grade encephalitis, which is marked by the presence of cysts in the brain, which may produce or induce production of a neurotransmitter, possibly dopamine, [21] therefore acting similarly to dopamine reuptake inhibitor type antidepressants and stimulants.
"In populations where this parasite is very common, mass personality modification could result in cultural change. [Variations in the prevalence of Toxoplasma gondii] may explain a substantial proportion of human population differences we see in cultural aspects that relate to ego, money, material possessions, work and rules." — Kevin Lafferty [22]
Correlations have been found between latent Toxoplasma infections and various characteristics: [12]
- Decreased novelty-seeking behaviour [23]
- Slower reactions
- Lower rule-consciousness and jealousy (in men) [23]
- More warmth and conscientiousness (in women) [23]
The evidence for behavioral effects on humans is relatively weak. There have been no randomized clinical trials studying the effects of toxoplasma on human behavior. Although some researchers have found potentially important associations with toxoplasma, it is possible that these associations merely reflect factors that predispose certain types of people to infection (e.g., people who exhibit risk-taking behaviors may be more likely to take the risk of eating undercooked meat).
Studies have found that toxoplasmosis is associated with an increased car accident rate, roughly doubling or tripling the chance of an accident relative to uninfected people.[21] [24] This may be due to the slowed reaction times that are associated with infection.[24] "If our data are true then about a million people a year die just because they are infected with toxoplasma," the researcher Jaroslav Flegr told The Guardian. [25] The data shows that the risk decreases with time after infection, but is not due to age.[21] Ruth Gilbert, medical coordinator of the European Multicentre Study on Congenital Toxoplasmosis, told BBC News Online these findings could be due to chance, or due to social and cultural factors associated with toxoplasma infection. [26]However there is also evidence of a delayed effect which increases reaction times.[27]
Other studies suggest that the parasite may influence personality. There are claims of toxoplasma causing antisocial attitudes in men and promiscuity [28] (or even "signs of higher intelligence" [29] ) in women, and greater susceptibility to schizophrenia and manic depression in all infected persons.[28] A 2004 study found that toxoplasma "probably induce[s] a decrease of novelty seeking." [30]
According to Sydney University of Technology infectious disease researcher Nicky Boulter in an article that appeared in the January/February 2007 edition of Australasian Science magazine, Toxoplasma infections lead to changes depending on the sex of the infected person. [31]
The study suggests that male carriers have lower IQs, a tendency to achieve a lower level of education and have shorter attention spans, a greater likelihood of breaking rules and taking risks, and are more independent, anti-social, suspicious, jealous and morose. It also suggests that these men are deemed less attractive to women. Women carriers are suggested to be more outgoing, friendly, more promiscuous, and are considered more attractive to men compared with non-infected controls.
Toxoplasma's role in schizophrenia
The possibility that toxoplasmosis is one cause of schizophrenia has been studied by scientists since at least 1953. [32] These studies had attracted little attention from U.S. researchers until they were publicized through the work of prominent psychiatrist and advocate E. Fuller Torrey. In 2003, Torrey published a review of this literature, reporting that almost all the studies had found that schizophrenics have elevated rates of toxoplasma infection.[32] A 2006 paper has even suggested that prevalence of toxoplasmosis has large-scale effects on national culture. [33] These types of studies are suggestive but cannot confirm a causal relationship (because of the possibility, for example, that schizophrenia increases the likelihood of toxoplasma infection rather than the other way around).[32]
- Acute Toxoplasma infection sometimes leads to psychotic symptoms not unlike schizophrenia.
- Some anti-psychotic medications that are used to treat schizophrenia, such as Haloperidol, also stop the growth of Toxoplasma in cell cultures.
- Several studies have found significantly higher levels of Toxoplasma antibodies in schizophrenia patients compared to the general population.[34]
- Toxoplasma infection causes damage to astrocytes in the brain, and such damage is also seen in schizophrenia.
Laboratory Findings
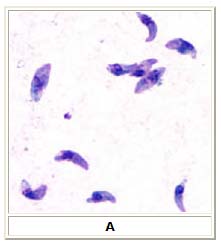
A: Toxoplasma gondii tachyzoites, stained with Giemsa, from a smear of peritoneal fluid obtained from a mouse inoculated with T. gondii. Tachyzoites are typically crescent shaped with a prominent, centrally placed nucleus.
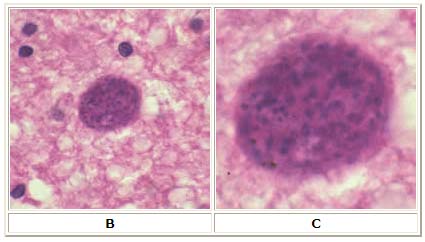
B: Toxoplasma gondii cyst in brain tissue stained with hematoxylin and eosin (100×).
C: Zoom of Image B, T. gondii cyst.
Treatment
Treatment is not needed for a healthy person who is not pregnant. Symptoms will usually go away within a few weeks. Treatment may be recommended for pregnant women or persons who have weakened immune systems [35].
Pharmacotherapy
Medications that are prescribed for acute Toxoplasmosis are:
- Pyrimethamine — an antimalarial medication.
- Sulfadiazine — an antibiotic used in combination with pyrimethamine to treat toxoplasmosis.
- clindamycin — an antibiotic. This is used most often for people with HIV/AIDS.
- spiramycin — another antibiotic. This is used most often for pregnant women to prevent the infection of their child.
(Other antibiotics such as minocycline have seen some use as a salvage therapy).
Medications that are prescribed for latent Toxoplasmosis are:
- atovaquone — an antibiotic that has been used to kill Toxoplasma cysts in situ in AIDS patients. [36]
- clindamycin — an antibiotic which, in combination with atovaquone, seemed to optimally kill cysts in mice.[37]
However, in latent infections successful treatment is not guaranteed, and some subspecies exhibit resistance.
Prevention
There are several general sanitation and food safety steps to reduce the chances of becoming infected with Toxoplasma:
- Gloves should be worn when gardening or doing anything outdoors that involves handling soil. Cats, which may pass the parasite in their feces, often use gardens and sandboxes as litter boxes. Hands should be washed well with soap and water after outdoor activities, especially before you eat or prepare any food.
- When preparing raw meat, cutting boards, sinks, knives, and other utensils that might have touched the raw meat should be washed thoroughly with soap and hot water to avoid cross-contaminating other foods. Hands should be washed well with soap and water after handling raw meat.
- All meat should be cooked thoroughly; that is, to an internal temperature of 160° F
Pregnancy Precautions
For congenital toxoplasmosis, a special form in which an unborn child is infected via the placenta, a positive antibody titer indicates previous exposure and immunity and largely ensures the unborn baby's safety. If a woman receives her first exposure to toxoplasmosis while pregnant, the baby is at particular risk. A woman with no previous exposure should avoid handling raw meat, exposure to cat faeces, and gardening (cat faeces are common in garden soil). Most cats are not actively shedding oocysts and so are not a danger, but the risk may be reduced further by having the litterbox emptied daily (oocysts require longer than a single day to become infective), and by having someone else empty the litterbox.
Acknowledgements
The content on this page was first contributed by: C. Michael Gibson, M.S., M.D.
References
- ↑ Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed. ed.). McGraw Hill. pp. pp. 723&ndash, 7. ISBN 0838585299.
- ↑ Torda A (2001). "Toxoplasmosis. Are cats really the source?". Aust Fam Physician. 30 (8): 743–7. PMID 11681144.
- ↑ Montoya J, Liesenfeld O (2004). "Toxoplasmosis". Lancet. 363 (9425): 1965–76. PMID 15194258.
- ↑ McQuillan G, Kruszon-Moran D, Kottiri B, Curtin L, Lucas J, Kington R (2004). "Racial and ethnic differences in the seroprevalence of 6 infectious diseases in the United States: data from NHANES III, 1988-1994". Am J Public Health. 94 (11): 1952–8. PMID 15514236.
- ↑ 5.0 5.1 Sukthana Y (2006). "Toxoplasmosis: beyond animals to humans". TRENDS in Parisitology. 22 (3): 137.
- ↑ http://www.pamf.org/serology/clinicianguide.html#toxosero
- ↑ http://www.dpd.cdc.gov/dpdx/HTML/Toxoplasmosis.htm
- ↑ http://www.cdc.gov/ncidod/EID/vol9no11/03-0098.htm
- ↑ Jones J, Kruszon-Moran D, Wilson M (2003). "Toxoplasma gondii infection in the United States, 1999-2000". Emerg Infect Dis. 9 (11): 1371–4. PMID 14718078.
- ↑ David Adam, Guardian Unlimited. Can a parasite carried by cats change your personality?, 25 Sep. 2003
- ↑ Toxoplasmosis in the Netherlands by the Laboratory for Diagnoses for Infectious Diseases and Screening; RIVM Bilthoven [1]
- ↑ 12.0 12.1 Carl Zimmer, The Loom. A Nation of Neurotics? Blame the Puppet Masters?, 1 Aug. 2006
- ↑ http://www.cdc.gov/ncidod/dpd/parasites/toxoplasmosis/factsht_toxoplasmosis.htm
- ↑ http://www.dpd.cdc.gov/dpdx/HTML/Toxoplasmosis.htmhttp://www.cdc.gov/ncidod/dpd/parasites/toxoplasmosis/factsht_toxoplasmosis.htm
- ↑ 15.0 15.1 "Toxoplasmosis". Centers of Disease Control and Prevention. 2004-11-22.
- ↑ [2]
- ↑ http://www.dpd.cdc.gov/dpdx/HTML/Toxoplasmosis.htm
- ↑ Jaroslav Flegr. Women infected with parasite Toxoplasma have more sons, Naturwissenschaften, August 2006. full text
- ↑ Berdoy M, Webster J, Macdonald D (2000). Fatal Attraction in Rats Infected with Toxoplasma gondii. Proceedings of the Royal Society of London, B267:1591-1594. CiteULike
- ↑ {{cite news | title='Cat Box Disease' May Change Human Personality And Lower IQ | publisher=The Daily Telegraph | date=April 8, 2000
- ↑ 21.0 21.1 21.2 Flegr J, Havlíček J, Kodym P, Malý M, Šmahel Z (2002). "Increased risk of traffic accidents in subjects with latent toxoplasmosis: a retrospective case-control study". BMC Infect Dis. 2: 11. PMID 12095427.
- ↑ Kevin Lafferty [3]
- ↑ 23.0 23.1 23.2 ">Jaroslav Flegr (2007). "Effects of Toxoplasma on Human Behaviour". Schizophrenia Bulletin. 33 (3): 757–760. Unknown parameter
|month=ignored (help) - ↑ 24.0 24.1 Yereli K, Balcioglu IC, Ozbilgin A. (2005). "Is Toxoplasma gondii a potential risk for traffic accidents in Turkey?". Forensic Sci Int. PMID 16332418. Unknown parameter
|month=ignored (help) - ↑ "Can a parasite carried by cats change your personality?". The Guardian. September 25, 2003.
- ↑ "Dirt infection link to car crashes". BBC News. August 10, 2002.
- ↑ J. Havlícek, Z. Gašová, A. P. Smith, K. Zvára and J. Flegr, Decrease of psychomotor performance in subjects with latent ‘asymptomatic’ toxoplasmosis, Parasitology (2001), 122: 515-520
- ↑ 28.0 28.1 "Dangerrrr: cats could alter your personality". Times Online. June 23, 2005.
- ↑ "Can a parasite carried by cats change your personality?". The Guardian. September 25, 2003.
- ↑ Novotná M, Hanusova J, Klose J, Preiss M, Havlicek J, Roubalová K, Flegr J (2004). "Probable neuroimmunological link between Toxoplasma and cytomegalovirus infections and personality changes in the human host". BMC Infect Dis. 5: 54. PMID 16000166. Unknown parameter
|month=ignored (help) - ↑ AAP, SMH Parasite makes men dumb, women sexy, 26 Dec. 2006
- ↑ 32.0 32.1 32.2 Torrey EF, Yolken RH (2003). "Toxoplasma gondii and schizophrenia". Emerging Infect. Dis. 9 (11): 1375–80. PMID 14725265.free full text
- ↑ Lafferty, Kevin D. (2006). "Can the common brain parasite, Toxoplasma gondii, influence human culture?". Proceedings of the Royal Society B: Biological Sciences (FirstCite Early Online Publishing). doi:10.1098/rspb.2006.3641. ISSN 0962-8452 (Paper) 1471-2954 (Online).
- ↑ Wang H, Wang G, Li Q, Shu C, Jiang M, Guo Y (2006). "Prevalence of Toxoplasma infection in first-episode schizophrenia and comparison between Toxoplasma-seropositive and Toxoplasma-seronegative schizophrenia". Acta Psychiatrica Scandinavica. 114 (1): 40–8. PMID 16774660.
- ↑ http://www.dpd.cdc.gov/dpdx/HTML/Toxoplasmosis.htm
- ↑ "Toxoplasmosis - treatment key research". NAM & aidsmap. 2005-11-02.
- ↑ Djurković-Djaković O, Milenković V, Nikolić A, Bobić B, Grujić J (2002). "Efficacy of atovaquone combined with clindamycin against murine infection with a cystogenic (Me49) strain of Toxoplasma gondii" (PDF). J Antimicrob Chemother. 50 (6): 981–7. doi:10.1093/jac/dkf251. PMID 12461021.
External links
- Parts of this article are taken from the public domain CDC factsheet: Toxoplasmosis
- Report on the link between Shizoprenia and Toxoplasmosis
Template:Protozoal diseases Template:SIB
ar:مقوسات
cs:Toxoplazmóza
de:Toxoplasmose
el:Τοξοπλάσμωση
hr:Toksoplazmoza
id:Toksoplasmosis
it:Toxoplasmosi
he:טוקסופלזמוזיס
hu:Toxoplazmózis
ms:Toxoplasmosis
nl:Toxoplasmose
no:Toksoplasmose
sk:Toxoplazmóza
fi:Toksoplasmoosi