Toprol XL nonclinical toxicology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 9: | Line 9: | ||
All genotoxicity tests performed on metoprolol tartrate (a dominant lethal study in mice, [[chromosome]] studies in [[somatic cell]]s, a [[Salmonella]]/mammalian-[[microsome]] mutagenicity test, and a nucleus anomaly test in somatic [[interphase]] nuclei) and metoprolol succinate (a [[Salmonella]]/mammalian-[[microsome]] mutagenicity test) were negative. | All genotoxicity tests performed on metoprolol tartrate (a dominant lethal study in mice, [[chromosome]] studies in [[somatic cell]]s, a [[Salmonella]]/mammalian-[[microsome]] mutagenicity test, and a nucleus anomaly test in somatic [[interphase]] nuclei) and metoprolol succinate (a [[Salmonella]]/mammalian-[[microsome]] mutagenicity test) were negative. | ||
No evidence of impaired fertility due to metoprolol tartrate was observed in a study performed in rats at doses up to 22 times, on a mg/m<sup>2</sup> basis, the daily dose of 200 mg in a 60 | No evidence of impaired fertility due to metoprolol tartrate was observed in a study performed in rats at doses up to 22 times, on a mg/m<sup>2</sup> basis, the daily dose of 200 mg in a 60 kg patient.<ref name="dailymed.nlm.nih.gov">{{Cite web | last = | first = | title = TOPROL XL (METOPROLOL SUCCINATE) TABLET, EXTENDED RELEASE [BRYANT RANCH PREPACK] | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=01038198-b4f0-41f3-9a9c-5c84e5a0d3b9 | publisher = | date = | accessdate = }}</ref> | ||
==References== | ==References== | ||
Revision as of 22:32, 13 March 2014
 | |
| Clinical data | |
|---|---|
| Trade names | Lopressor, Toprol-xl |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682864 |
| [[Regulation of therapeutic goods |Template:Engvar data]] |
|
| Pregnancy category | |
| Routes of administration | Oral, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 12% |
| Metabolism | Hepatic via CYP2D6, CYP3A4 |
| Elimination half-life | 3-7 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
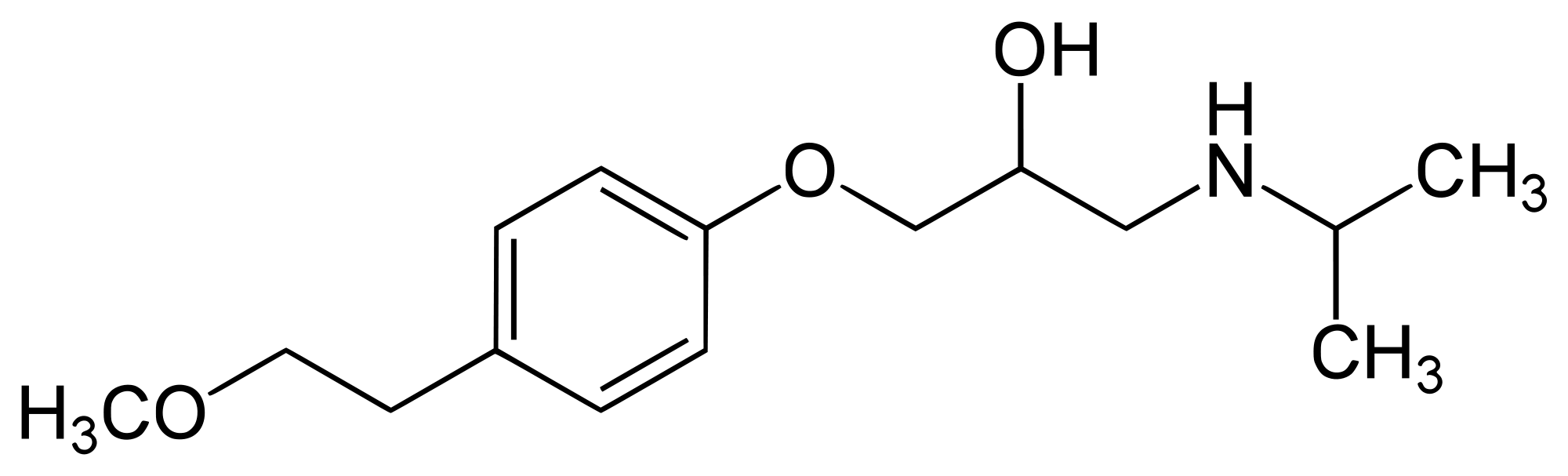
| Formula | C15H25NO3 |
| Molar mass | 267.364 g/mol |
| 3D model (JSmol) | |
| Melting point | 120 °C (248 °F) |
| |
| |
| (verify) | |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have been conducted to evaluate the carcinogenic potential of metoprolol tartrate. In 2-year studies in rats at three oral dosage levels of up to 800 mg/kg/day (41 times, on a mg/m2 basis, the daily dose of 200 mg for a 60-kg patient), there was no increase in the development of spontaneously occurring benign or malignant neoplasms of any type. The only histologic changes that appeared to be drug related were an increased incidence of generally mild focal accumulation of foamy macrophages in pulmonary alveoli and a slight increase in biliary hyperplasia. In a 21-month study in Swiss albino mice at three oral dosage levels of up to 750 mg/kg/day (18 times, on a mg/m2 basis, the daily dose of 200 mg for a 60-kg patient), benign lung tumors (small adenomas) occurred more frequently in female mice receiving the highest dose than in untreated control animals. There was no increase in malignant or total (benign plus malignant) lung tumors, nor in the overall incidence of tumors or malignant tumors. This 21-month study was repeated in CD-1 mice, and no statistically or biologically significant differences were observed between treated and control mice of either sex for any type of tumor.
All genotoxicity tests performed on metoprolol tartrate (a dominant lethal study in mice, chromosome studies in somatic cells, a Salmonella/mammalian-microsome mutagenicity test, and a nucleus anomaly test in somatic interphase nuclei) and metoprolol succinate (a Salmonella/mammalian-microsome mutagenicity test) were negative.
No evidence of impaired fertility due to metoprolol tartrate was observed in a study performed in rats at doses up to 22 times, on a mg/m2 basis, the daily dose of 200 mg in a 60 kg patient.[1]
References
Adapted from the FDA Package Insert.
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Infobox drug articles with non-default infobox title
- Beta blockers
- Cardiovascular Drugs
- Drugs