Tolterodine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2], Rabin Bista, M.B.B.S. [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Tolterodine is a cholinergic muscarinic antagonist that is FDA approved for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency. Common adverse reactions include abdominal pain, constipation, xerostomia, headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Overactive Bladder
- Dosing information
- Initial recommended dose: 2 mg PO bid.
- The dose may be lowered to 1 mg twice daily based on individual response and tolerability.
- For patients with significantly reduced hepatic or renal function or who are currently taking drugs that are potent inhibitors of CYP3A4
- Recommended dose: 1 mg PO bid
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of tolterodine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of tolterodine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The pharmacokinetics of tolterodine have not been established in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of tolterodine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of tolterodine in pediatric patients.
Contraindications
- DETROL tablets are contraindicated in patients with urinary retention, gastric retention, or uncontrolled narrow-angle glaucoma. DETROL is also contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients, or to fesoterodine fumarate extended-release tablets which, like DETROL, are metabolized to 5-hydroxymethyl tolterodine.
Warnings
- Anaphylaxis and angioedema requiring hospitalization and emergency medical treatment have occurred with the first or subsequent doses of DETROL. In the event of difficulty in breathing, upper airway obstruction, or fall in blood pressure, DETROL should be discontinued and appropriate therapy promptly provided.
Adverse Reactions
Clinical Trials Experience
- The Phase 2 and 3 clinical trial program for DETROL tablets included 3071 patients who were treated with DETROL (N=2133) or placebo (N=938). The patients were treated with 1, 2, 4, or 8 mg/day for up to 12 months. No differences in the safety profile of tolterodine were identified based on age, gender, race, or metabolism.
- The data described below reflect exposure to DETROL 2 mg bid in 986 patients and to placebo in 683 patients exposed for 12 weeks in five Phase 3, controlled clinical studies. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and approximating rates.
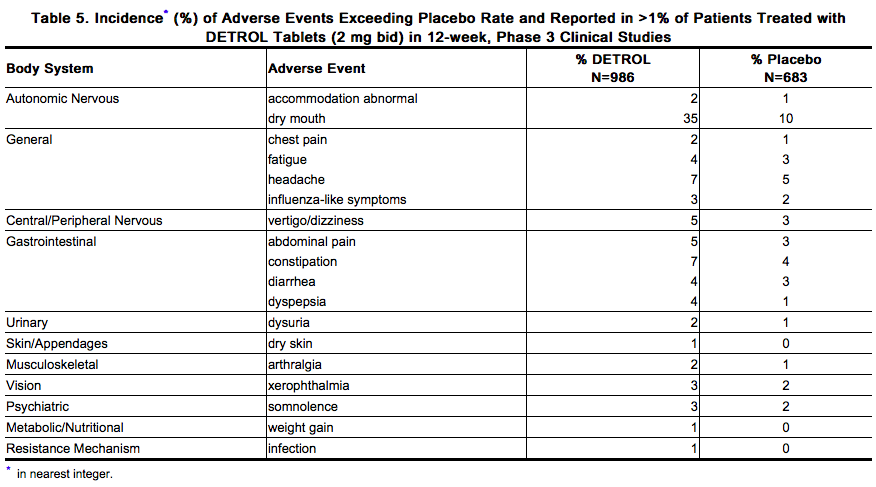
Sixty-six percent of patients receiving DETROL 2 mg bid reported adverse events versus 56% of placebo patients. The most common adverse events reported by patients receiving DETROL were dry mouth, headache, constipation, vertigo/dizziness, and abdominal pain. Dry mouth, constipation, abnormal vision (accommodation abnormalities), urinary retention, and xerophthalmia are expected side effects of antimuscarinic agents. Dry mouth was the most frequently reported adverse event for patients treated with DETROL 2 mg bid in the Phase 3 clinical studies, occurring in 34.8% of patients treated with DETROL and 9.8% of placebo-treated patients. One percent of patients treated with DETROL discontinued treatment due to dry mouth. The frequency of discontinuation due to adverse events was highest during the first 4 weeks of treatment. Seven percent of patients treated with DETROL 2 mg bid discontinued treatment due to adverse events versus 6% of placebo patients. The most common adverse events leading to discontinuation of DETROL were dizziness and headache.
- Three percent of patients treated with DETROL 2 mg bid reported a serious adverse event versus 4% of placebo patients. Significant ECG changes in QT and QTc have not been demonstrated in clinical-study patients treated with DETROL 2 mg bid. Table 5 lists the adverse events reported in 1% or more of the patients treated with DETROL 2 mg bid in the 12-week studies. The adverse events are reported regardless of causality.
Postmarketing Experience
- The following events have been reported in association with tolterodine use in worldwide post-marketing experience:
General: anaphylaxis and angioedema;
Cardiovascular: tachycardia, palpitations, peripheral edema;
Central/Peripheral Nervous: confusion, disorientation, memory impairment, hallucinations.
- Reports of aggravation of symptoms of dementia (e.g., confusion, disorientation, delusion) have been reported after tolterodine therapy was initiated in patients taking cholinesterase inhibitors for the treatment of dementia.
- Because these spontaneously reported events are from the worldwide post-marketing experience, the frequency of events and the role of tolterodine in their causation cannot be reliably determined.
Drug Interactions
Fluoxetine
- Fluoxetine is a selective serotonin reuptake inhibitor and a potent inhibitor of CYP2D6 activity. In a study to assess the effect of fluoxetine on the pharmacokinetics of tolterodine immediate release and its metabolites, it was observed that fluoxetine significantly inhibited the metabolism of tolterodine immediate release in extensive metabolizers, resulting in a 4.8-fold increase in tolterodine AUC. There was a 52% decrease in Cmax and a 20% decrease in AUC of the 5-hydroxymethyl metabolite. Fluoxetine thus alters the pharmacokinetics in patients who would otherwise be extensive metabolizers of tolterodine immediate release to resemble the pharmacokinetic profile in poor metabolizers. The sums of unbound serum concentrations of tolterodine immediate release and the 5-hydroxymethyl metabolite are only 25% higher during the interaction. No dose adjustment is required when DETROL and fluoxetine are coadministered.
Other Drugs Metabolized by Cytochrome P450 Isoenzymes
- Tolterodine immediate release does not cause clinically significant interactions with other drugs metabolized by the major drug metabolizing CYP enzymes. In vivo drug-interaction data show that tolterodine immediate release does not result in clinically relevant inhibition of CYP1A2, 2D6, 2C9, 2C19, or 3A4 as evidenced by lack of influence on the marker drugs caffeine, debrisoquine, S-warfarin, and omeprazole. In vitro data show that tolterodine immediate release is a competitive inhibitor of CYP2D6 at high concentrations (Ki 1.05 µM), while tolterodine immediate release as well as the 5-hydroxymethyl metabolite are devoid of any significant inhibitory potential regarding the other isoenzymes.
CYP3A4 Inhibitors
- The effect of 200 mg daily dose of ketoconazole on the pharmacokinetics of tolterodine immediate release was studied in 8 healthy volunteers, all of whom were poor metabolizers (see Pharmacokinetics,Variability in Metabolism for discussion of poor metabolizers). In the presence of ketoconazole, the mean Cmax and AUC of tolterodine increased by 2 and 2.5 fold, respectively. Based on these findings, other potent CYP3A inhibitors such as other azole antifungals (eg, itraconazole, miconazole) or macrolide antibiotics (eg, erythromycin, clarithromycin) or cyclosporine or vinblastine may also lead to increases of tolterodine plasma concentrations .
Warfarin
- In healthy volunteers, co administration of tolterodine immediate release 4 mg (2 mg bid) for 7 days and a single dose of warfarin 25 mg on day 4 had no effect on prothrombin time, factor VII suppression, or on the pharmacokinetics of warfarin.
Oral Contraceptives
- Tolterodine immediate release 4 mg (2 mg bid) had no effect on the pharmacokinetics of an oral contraceptive (ethinyl estradiol 30 µg/levonorgestrel 150 µg) as evidenced by the monitoring of ethinyl estradiol and levonorgestrel over a 2-month cycle in healthy female volunteers.
Diuretics
- Co administration of tolterodine immediate release up to 8 mg (4 mg bid) for up to 12 weeks with diuretic agents, such as indapamide, hydrochlorothiazide, triamterene, bendroflumethiazide, chlorothiazide, methylchlorothiazide, or furosemide, did not cause any adverse electrocardiographic (ECG) effects.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Pregnancy Category C
- At oral doses of 20 mg/kg/day (approximately 14 times the human exposure), no anomalies or malformations were observed in mice. When given at doses of 30 to 40 mg/kg/day, tolterodine has been shown to be embryolethal, reduce fetal weight, and increase the incidence of fetal abnormalities (cleft palate, digital abnormalities, intra-abdominal hemorrhage, and various skeletal abnormalities, primarily reduced ossification) in mice. At these doses, the AUC values were about 20- to 25-fold higher than in humans. Rabbits treated subcutaneously at a dose of 0.8 mg/kg/day achieved an AUC of 100 µg∙h/L, which is about 3-fold higher than that resulting from the human dose. This dose did not result in any embryotoxicity or teratogenicity. There are no studies of tolterodine in pregnant women. Therefore, DETROL should be used during pregnancy only if the potential benefit for the mother justifies the potential risk to the fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Tolterodine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Tolterodine during labor and delivery.
Nursing Mothers
Tolterodine is excreted into the milk in mice. Offspring of female mice treated with tolterodine 20 mg/kg/day during the lactation period had slightly reduced body weight gain. The offspring regained the weight during the maturation phase. It is not known whether tolterodine is excreted in human milk; therefore, DETROL should not be administered during nursing. A decision should be made whether to discontinue nursing or to discontinue DETROL in nursing mothers.
Pediatric Use
Efficacy in the pediatric population has not been demonstrated.
- Two pediatric phase 3 randomized, placebo-controlled, double-blind, 12-week studies were conducted using tolterodine extended release (DETROL LA) capsules. A total of 710 pediatric patients (486 on DETROL LA and 224 on placebo) aged 5–10 years with urinary frequency and urge urinary incontinence were studied. The percentage of patients with urinary tract infections was higher in patients treated with DETROL LA (6.6%) compared to patients who received placebo (4.5%). Aggressive, abnormal and hyperactive behavior and attention disorders occurred in 2.9% of children treated with DETROL LA compared to 0.9% of children treated with placebo.
Geriatic Use
- Of the 1120 patients who were treated in the four Phase 3, 12-week clinical studies of DETROL, 474 (42%) were 65 to 91 years of age. No overall differences in safety were observed between the older and younger patients.
Gender
There is no FDA guidance on the use of Tolterodine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Tolterodine with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Tolterodine in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Tolterodine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Tolterodine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Tolterodine in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
FDA package insert for tolterodine contains no information regarding drug monitoring.
IV Compatibility
There is limited information about the IV compatibility.
Overdosage
- A 27-month-old child who ingested 5 to 7 DETROL Tablets 2 mg was treated with a suspension of activated charcoal and was hospitalized overnight with symptoms of dry mouth. The child fully recovered.
Management of Overdosage
- Overdosage with DETROL can potentially result in severe central anticholinergic effects and should be treated accordingly.
ECG monitoring is recommended in the event of overdosage. In dogs, changes in the QT interval (slight prolongation of 10% to 20%) were observed at a suprapharmacologic dose of 4.5 mg/kg, which is about 68 times higher than the recommended human dose. In clinical trials of normal volunteers and patients, QT interval prolongation was observed with tolterodine immediate release at doses up to 8 mg (4 mg bid) and higher doses were not evaluated
Pharmacology
| |
Tolterodine
| |
| Systematic (IUPAC) name | |
| (R)-2-[3-(diisopropylamino)-1-phenylpropyl]-4-methylphenol | |
| Identifiers | |
| CAS number | |
| ATC code | G04 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 325.488 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 77% |
| Protein binding | Approximately 96.3%. |
| Metabolism | ? |
| Half life | 1.9-3.7 hours |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) ?(CA) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | ? |
Mechanism of Action
- FDA package insert for tolterodine contains no information regarding mechanism of action.
Structure
- DETROL Tablets contain tolterodine tartrate. The active moiety, tolterodine, is a muscarinic receptor antagonist. The chemical name of tolterodine tartrate is (R)-2-[3-[bis(1-methylethyl)-amino]1-phenylpropyl]-4-methylphenol [R-(R*,R*)]-2,3dihydroxybutanedioate (1:1) (salt). The empirical formula of tolterodine tartrate is C26H37NO7, and its molecular weight is 475.6. The structural formula of tolterodine tartrate is represented below:
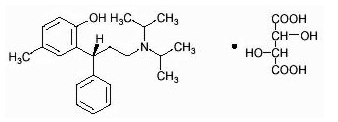
- Tolterodine tartrate is a white, crystalline powder. The pKa value is 9.87 and the solubility in water is 12 mg/mL. It is soluble in methanol, slightly soluble in ethanol, and practically insoluble in toluene. The partition coefficient (Log D) between n-octanol and water is 1.83 at pH 7.3.
DETROL Tablets for oral administration contain 1 or 2 mg of tolterodine tartrate. The inactive ingredients are colloidal anhydrous silica, calcium hydrogen phosphate dihydrate, cellulose microcrystalline, hypromellose, magnesium stearate, sodium starch glycolate (pH 3.0 to 5.0), stearic acid, and titanium dioxide.
Pharmacodynamics
FDA package insert for tolterodine contains no information regarding pharmacodynamics.
Pharmacokinetics
Absorption
- In a study with 14C-tolterodine solution in healthy volunteers who received a 5-mg oral dose, at least 77% of the radiolabeled dose was absorbed. Tolterodine immediate release is rapidly absorbed, and maximum serum concentrations (Cmax) typically occur within 1 to 2 hours after dose administration. Cmax and area under the concentration-time curve (AUC) determined after dosage of tolterodine immediate release are dose-proportional over the range of 1 to 4 mg.
Effect of Food
- Food intake increases the bioavailability of tolterodine (average increase 53%), but does not affect the levels of the 5-hydroxymethyl metabolite in extensive metabolizers. This change is not expected to be a safety concern and adjustment of dose is not needed.
Distribution
- Tolterodine is highly bound to plasma proteins, primarily α1-acid glycoprotein. Unbound concentrations of tolterodine average 3.7% ± 0.13% over the concentration range achieved in clinical studies. The 5-hydroxymethyl metabolite is not extensively protein bound, with unbound fraction concentrations averaging 36% ± 4.0%. The blood to serum ratio of tolterodine and the 5-hydroxymethyl metabolite averages 0.6 and 0.8, respectively, indicating that these compounds do not distribute extensively into erythrocytes. The volume of distribution of tolterodine following administration of a 1.28-mg intravenous dose is 113 ± 26.7 L.
Metabolism
- Tolterodine is extensively metabolized by the liver following oral dosing. The primary metabolic route involves the oxidation of the 5-methyl group and is mediated by the cytochrome P450 2D6 (CYP2D6) and leads to the formation of a pharmacologically active 5-hydroxymethyl metabolite. Further metabolism leads to formation of the 5-carboxylic acid and N-dealkylated 5-carboxylic acid metabolites, which account for 51% ± 14% and 29% ± 6.3% of the metabolites recovered in the urine, respectively.
Variability in Metabolism
- A subset (about 7%) of the population is devoid of CYP2D6, the enzyme responsible for the formation of the 5-hydroxymethyl metabolite of tolterodine. The identified pathway of metabolism for these individuals ("poor metabolizers") is dealkylation via cytochrome P450 3A4 (CYP3A4) to N-dealkylated tolterodine. The remainder of the population is referred to as "extensive metabolizers." Pharmacokinetic studies revealed that tolterodine is metabolized at a slower rate in poor metabolizers than in extensive metabolizers; this results in significantly higher serum concentrations of tolterodine and in negligible concentrations of the 5-hydroxymethyl metabolite.
Excretion
- Following administration of a 5-mg oral dose of 14C-tolterodine solution to healthy volunteers, 77% of radioactivity was recovered in urine and 17% was recovered in feces in 7 days. Less than 1% (<2.5% in poor metabolizers) of the dose was recovered as intact tolterodine, and 5% to 14% (<1% in poor metabolizers) was recovered as the active 5-hydroxymethyl metabolite.
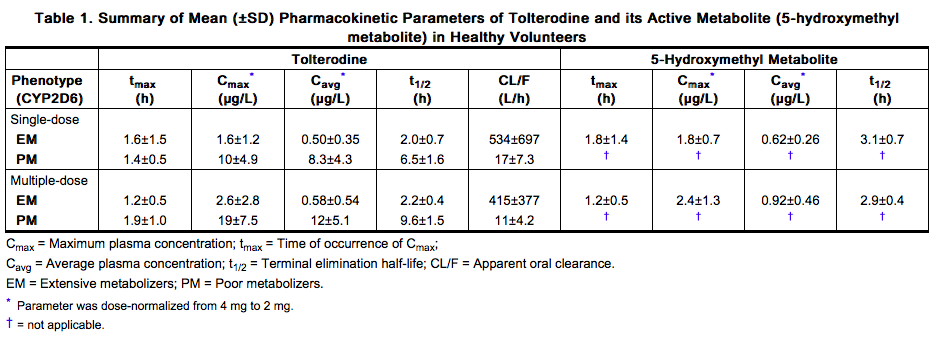
- A summary of mean (± standard deviation) pharmacokinetic parameters of tolterodine immediate release and the 5-hydroxymethyl metabolite in extensive (EM) and poor (PM) metabolizers is provided in Table 1. These data were obtained following single and multiple doses of tolterodine 4 mg administered twice daily to 16 healthy male volunteers (8 EM, 8 PM).
Pharmacokinetics in Special Populations
Age
- In Phase 1, multiple-dose studies in which tolterodine immediate release 4 mg (2 mg bid) was administered, serum concentrations of tolterodine and of the 5-hydroxymethyl metabolite were similar in healthy elderly volunteers (aged 64 through 80 years) and healthy young volunteers (aged less than 40 years). In another Phase 1 study, elderly volunteers (aged 71 through 81 years) were given tolterodine immediate release 2 or 4 mg (1 or 2 mg bid). Mean serum concentrations of tolterodine and the 5-hydroxymethyl metabolite in these elderly volunteers were approximately 20% and 50% higher, respectively, than reported in young healthy volunteers. However, no overall differences were observed in safety between older and younger patients on tolterodine in Phase 3, 12-week, controlled clinical studies; therefore, no tolterodine dosage adjustment for elderly patients is recommended .
Pediatric
- The pharmacokinetics of tolterodine have not been established in pediatric patients.
Gender
- The pharmacokinetics of tolterodine immediate release and the 5-hydroxymethyl metabolite are not influenced by gender. Mean Cmax of tolterodine (1.6 µg/L in males versus 2.2 µg/L in females) and the active 5-hydroxymethyl metabolite (2.2 µg/L in males versus 2.5 µg/L in females) are similar in males and females who were administered tolterodine immediate release 2 mg. Mean AUC values of tolterodine (6.7 µg∙h/L in males versus 7.8 µg∙h/L in females) and the 5-hydroxymethyl metabolite (10 µg∙h/L in males versus 11 µg∙h/L in females) are also similar. The elimination half-life of tolterodine for both males and females is 2.4 hours, and the half-life of the 5-hydroxymethyl metabolite is 3.0 hours in females and 3.3 hours in males.
Race
- Pharmacokinetic differences due to race have not been established.
Renal Insufficiency
- Renal impairment can significantly alter the disposition of tolterodine immediate release and its metabolites. In a study conducted in patients with creatinine clearance between 10 and 30 mL/min, tolterodine immediate release and the 5-hydroxymethyl metabolite levels were approximately 2–3 fold higher in patients with renal impairment than in healthy volunteers. Exposure levels of other metabolites of tolterodine (e.g., tolterodine acid, N-dealkylated tolterodine acid, N-dealkylated tolterodine, and N-dealkylated hydroxylated tolterodine) were significantly higher (10–30 fold) in renally impaired patients as compared to the healthy volunteers. The recommended dosage for patients with significantly reduced renal function is DETROL 1 mg twice daily .
Hepatic Insufficiency
- Liver impairment can significantly alter the disposition of tolterodine immediate release. In a study conducted in cirrhotic patients, the elimination half-life of tolterodine immediate release was longer in cirrhotic patients (mean, 7.8 hours) than in healthy, young, and elderly volunteers (mean, 2 to 4 hours). The clearance of orally administered tolterodine was substantially lower in cirrhotic patients (1.0 ± 1.7 L/h/kg) than in the healthy volunteers (5.7 ± 3.8 L/h/kg). The recommended dose for patients with significantly reduced hepatic function is DETROL 1 mg twice daily
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity studies with tolterodine were conducted in mice and rats. At the maximum tolerated dose in mice (30 mg/kg/day), female rats (20 mg/kg/day), and male rats (30 mg/kg/day), AUC values obtained for tolterodine were 355, 291, and 462 µg∙h/L, respectively. In comparison, the human AUC value for a 2-mg dose administered twice daily is estimated at 34 µg∙h/L. Thus, tolterodine exposure in the carcinogenicity studies was 9- to 14-fold higher than expected in humans. No increase in tumors was found in either mice or rats.
- No mutagenic effects of tolterodine were detected in a battery of in vitro tests, including bacterial mutation assays (Ames test) in 4 strains of Salmonella typhimurium and in 2 strains of Escherichia coli, a gene mutation assay in L5178Y mouse lymphoma cells, and chromosomal aberration tests in human lymphocytes. Tolterodine was also negative in vivo in the bone marrow micronucleus test in the mouse.
- In female mice treated for 2 weeks before mating and during gestation with 20 mg/kg/day (corresponding to AUC value of about 500 µg∙h/L), neither effects on reproductive performance or fertility were seen. Based on AUC values, the systemic exposure was about 15-fold higher in animals than in humans. In male mice, a dose of 30 mg/kg/day did not induce any adverse effects on fertility.
Clinical Studies
- DETROL Tablets were evaluated for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and frequency in four randomized, double-blind, placebo-controlled, 12-week studies. A total of 853 patients received DETROL 2 mg twice daily and 685 patients received placebo. The majority of patients were Caucasian (95%) and female (78%), with a mean age of 60 years (range, 19 to 93 years). At study entry, nearly all patients perceived they had urgency and most patients had increased frequency of micturitions and urge incontinence. These characteristics were well balanced across treatment groups for the studies.
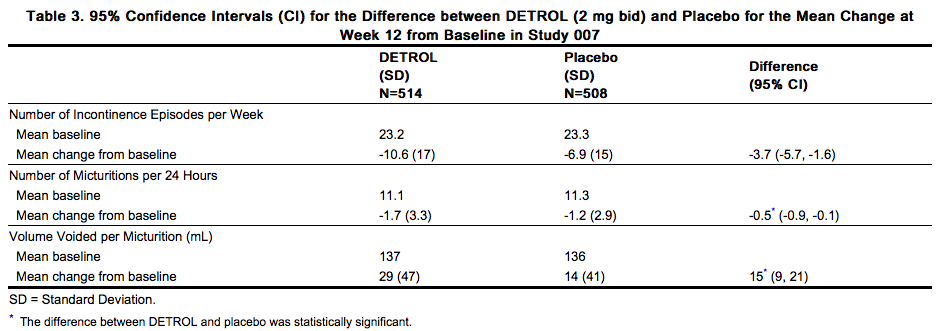
The efficacy endpoints for study 007 (see Table 3) included the change from baseline for:
- Number of incontinence episodes per week
- Number of micturitions per 24 hours (averaged over 7 days)
- Volume of urine voided per micturition (averaged over 2 days)
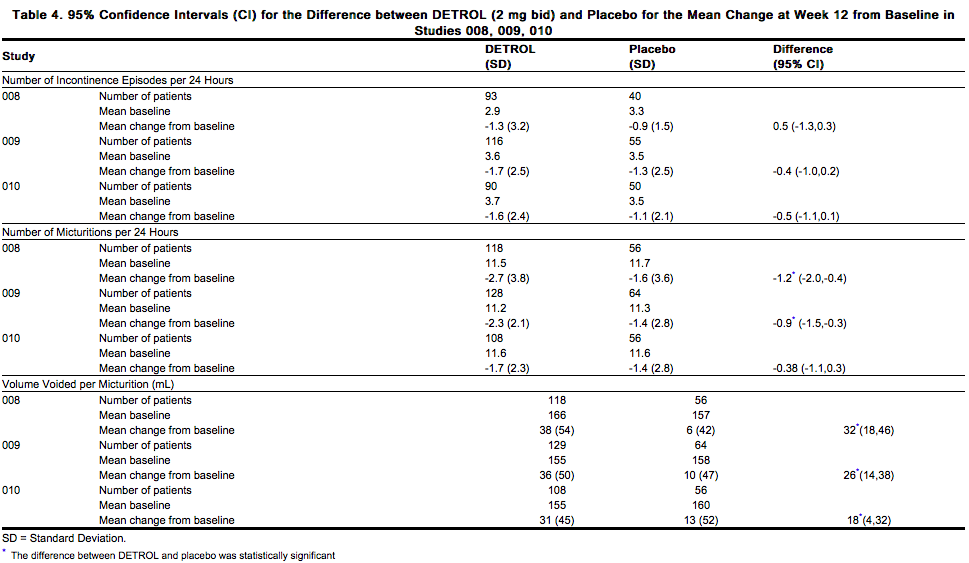
The efficacy endpoints for studies 008, 009, and 010 (see Table 4) were identical to the above endpoints with the exception that the number of incontinence episodes was per 24 hours (averaged over 7 days).
How Supplied
- DETROL Tablets 1 mg (white, round, biconvex, film-coated tablets engraved with arcs above and below the letters "TO") and DETROL Tablets 2 mg (white, round, biconvex, film-coated tablets engraved with arcs above and below the letters "DT") are supplied as follows:
Bottles of 60
- 1 mg NDC 0009-4541-02
- 2 mg NDC 0009-4544-02
Bottles of 500
- 1 mg NDC 0009-4541-03
- 2 mg NDC 0009-4544-03
Unit Dose Pack of 140
- 1 mg NDC 0009-4541-01
- 2 mg NDC 0009-4544-01
Storage
- Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature] (DTL).
Images
Drug Images
{{#ask: Page Name::Tolterodine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Tolterodine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information

Precautions with Alcohol
Alcohol-Tolterodine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Detrol
- Detrol LA
Look-Alike Drug Names
There is limited information about the look-alike drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Tolterodine |Pill Name=Tolterodine_1 mg_NDC 0009-4541.jpg |Drug Name=Tolterodine tartrate 1 MG Oral Tablet |Pill Ingred=cellulose, microcrystalline, hypromelloses, magnesium stearate, stearic acid, titanium dioxide, silicon dioxide, calcium phosphate, dibasic, anhydrous, sodium starch glycolate type b potato|+sep=; |Pill Imprint=TO |Pill Dosage=1 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=6.00 |Pill Scoring=1 |Pill Image= |Drug Author=TEVA Pharmaceuticals USA Inc |NDC=0009-4541
}}
{{#subobject:
|Page Name=Tolterodine |Pill Name=Tolterodine_2 mg_NDC 0009-4544.jpg |Drug Name=tolterodine tartrate tablet, film coated |Pill Ingred=silicon dioxide, cellulose, microcrystalline, hypromelloses, magnesium stearate, stearic acid, titanium dioxide|+sep=; |Pill Imprint=DT |Pill Dosage=2 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=6.00 |Pill Scoring=1 |Pill Image= |Drug Author=Pharmacia and Upjohn Company |NDC=0009-4544
}}
{{#subobject:
|Label Page=Tolterodine |Label Name=Tolterodine_label_01.jpg
}}
{{#subobject:
|Label Page=Tolterodine |Label Name=Tolterodine_label_02.jpg
}}
{{#subobject:
|Label Page=Tolterodine |Label Name=Tolterodine_label_03.jpg
}}
{{#subobject:
|Label Page=Tolterodine |Label Name=Tolterodine_label_04.jpg
}}
{{#subobject:
|Label Page=Tolterodine |Label Name=Tolterodine_label_05.jpg
}}
{{#subobject:
|Label Page=Tolterodine |Label Name=Tolterodine_label_06.jpg
}}
{{#subobject:
|Label Page=Tolterodine |Label Name=Tolterodine_panel_01.png
}}
{{#subobject:
|Label Page=Tolterodine |Label Name=Tolterodine_panel_02.png
}}