Thromboembolism: Difference between revisions
| Line 562: | Line 562: | ||
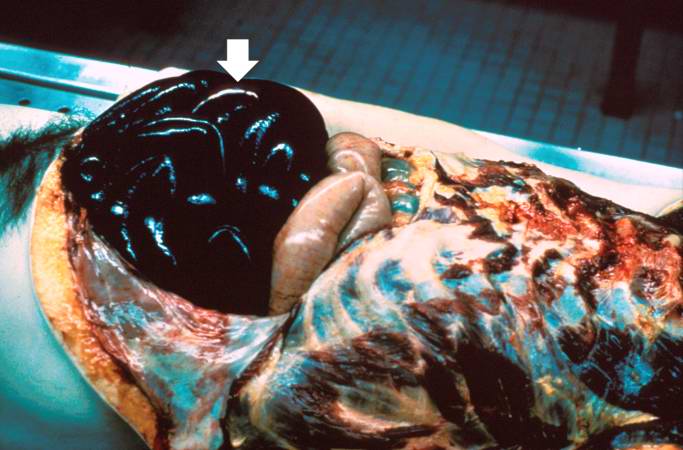
* A 67-year-old male was hospitalized because of extensive atherosclerotic cardiovascular disease. Following surgery, during which diseased portions of the femoral arteries were bypassed, he developed massive pulmonary embolism and expired. At autopsy, thrombi were found in the femoral and iliac veins, as well as in the larger pulmonary arteries. | * A 67-year-old male was hospitalized because of extensive atherosclerotic cardiovascular disease. Following surgery, during which diseased portions of the femoral arteries were bypassed, he developed massive pulmonary embolism and expired. At autopsy, thrombi were found in the femoral and iliac veins, as well as in the larger pulmonary arteries. | ||
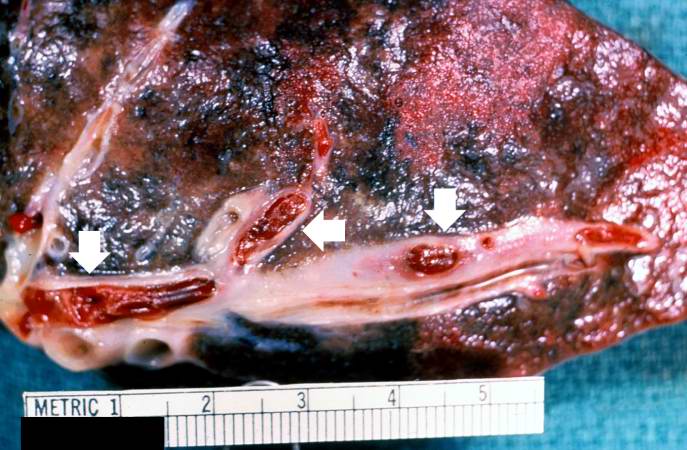
[[Image:Thromboembolus 1.jpg|left|thumb|400px|This is a gross photograph of a cut section of lung demonstrating thromboemboli in the pulmonary arteries (arrows).]] | |||
Image:Thromboembolus 1.jpg|This is a gross photograph of a cut section of lung demonstrating thromboemboli in the pulmonary arteries (arrows). | <br clear="left"/> | ||
</ | |||
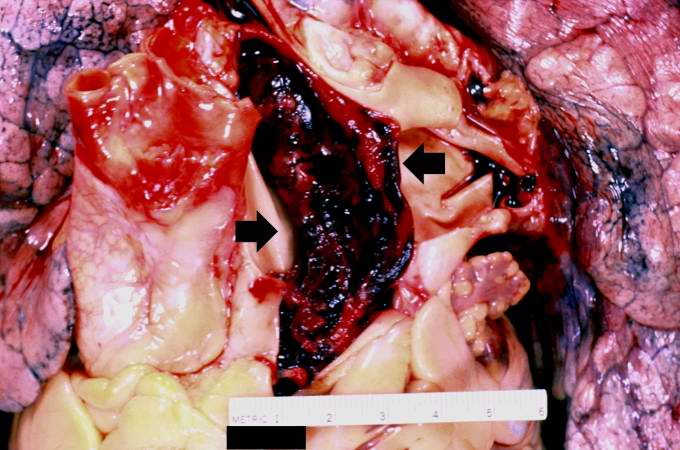
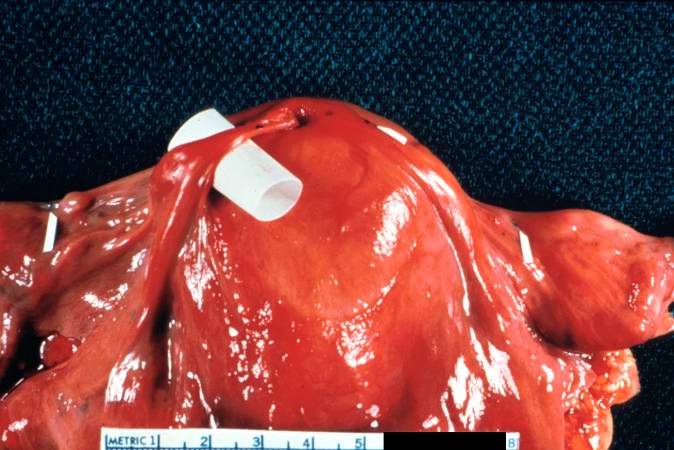
[[Image:Thromboembolus 2.jpg|left|thumb|400px|This is a gross photograph of the heart with the main pulmonary artery opened. Note the thromboembolus filling the pulmonary artery (arrows).]] | |||
<br clear="left"/> | |||
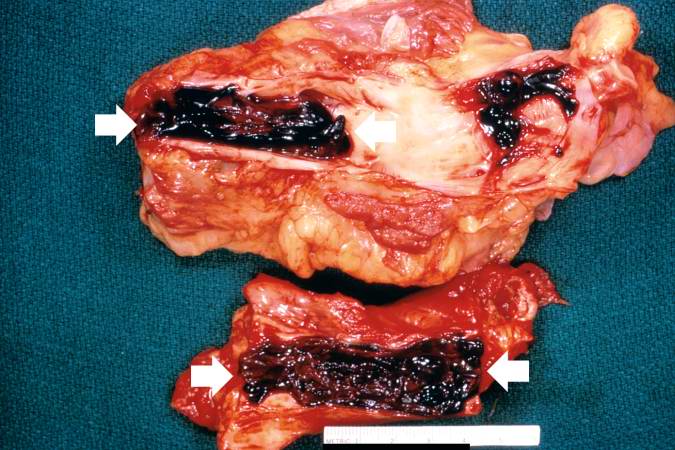
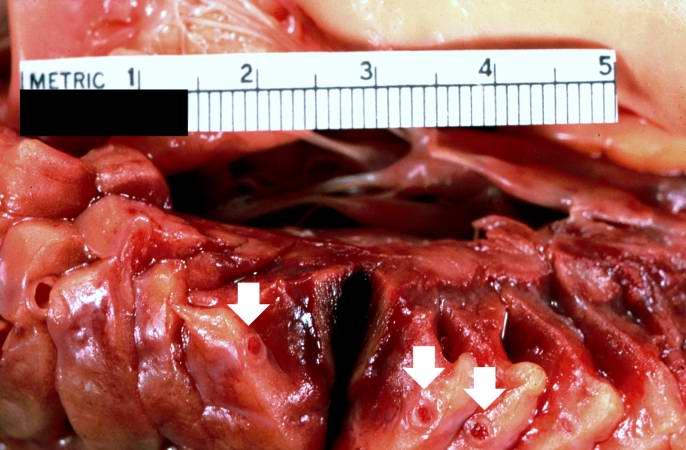
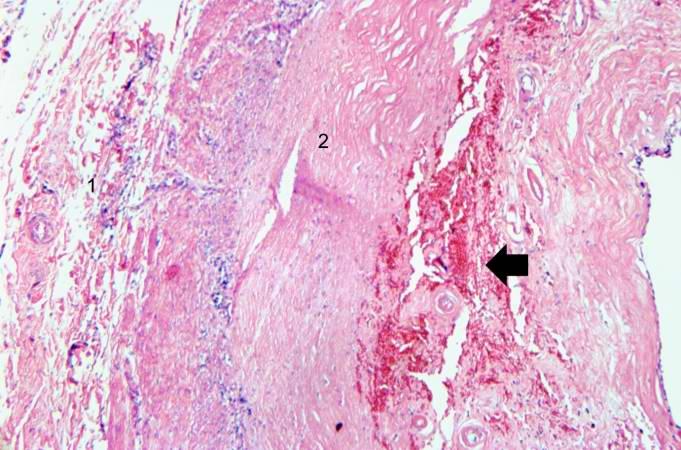
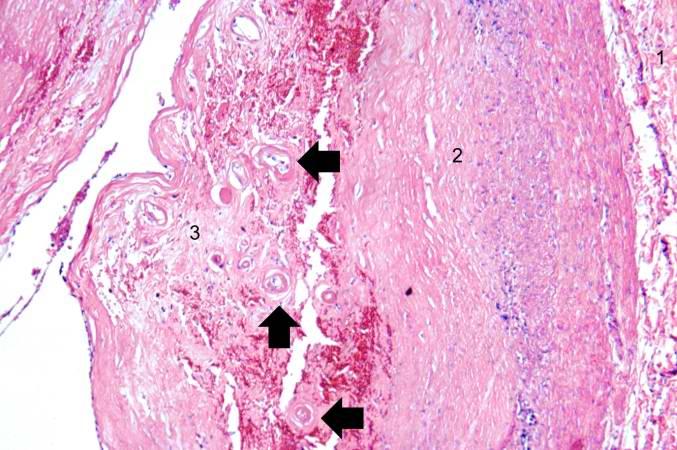
< | [[Image:Thromboembolus 3.jpg|left|thumb|400px|This is a gross photograph of portions of muscle from the legs including sections of leg veins. Note that the leg veins contain thrombus (arrows). ]] | ||
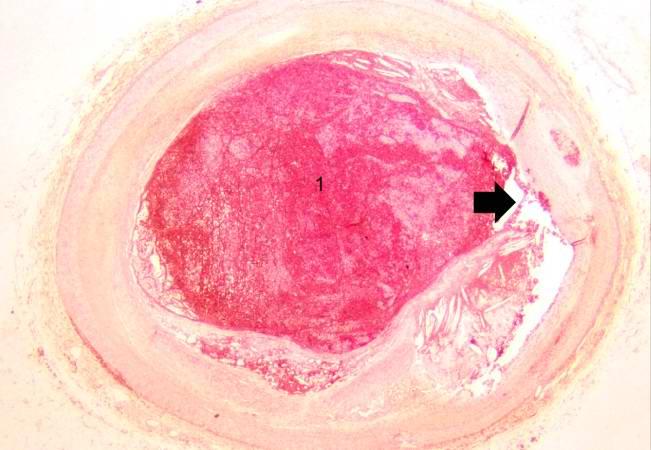
Image:Thromboembolus 4.jpg|This is a low-power photomicrograph of lung. A large thrombus is lodged at this branch point in the pulmonary artery. Note the hemorrhage and congestion in the surrounding lung parenchyma. | <br clear="left"/> | ||
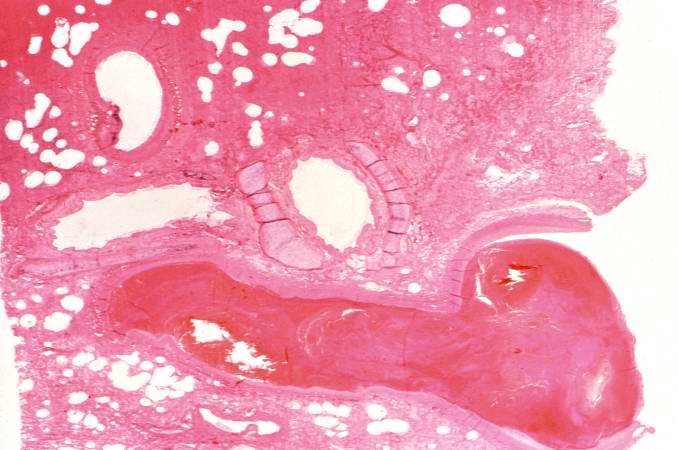
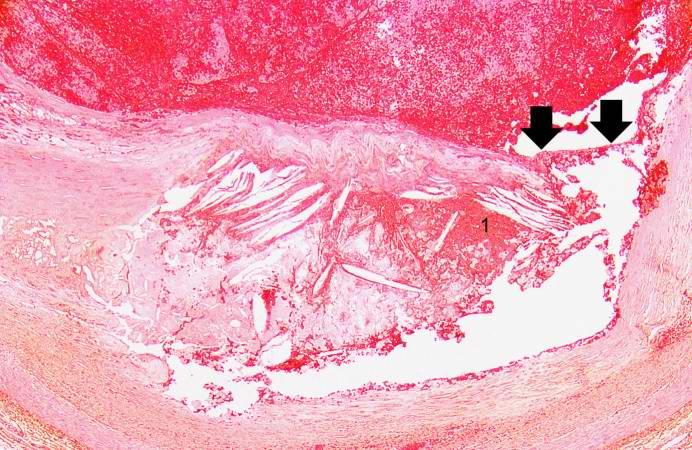
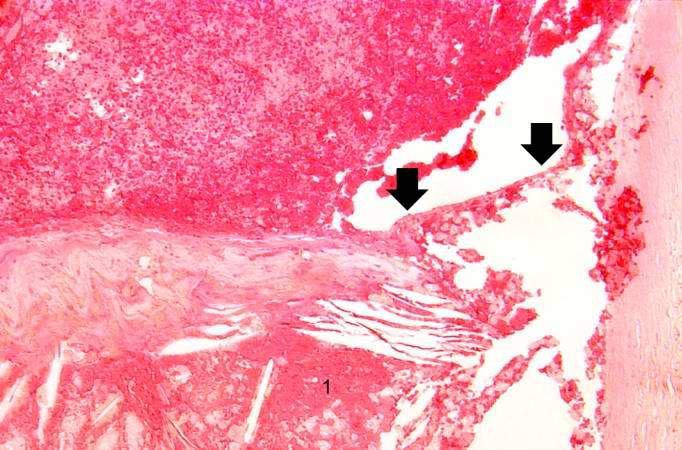
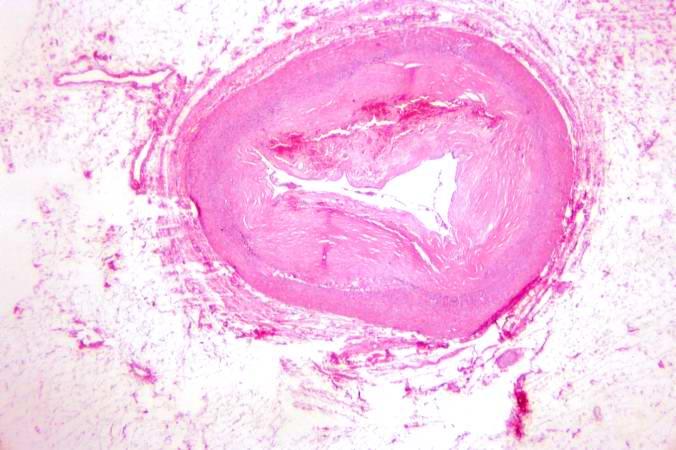
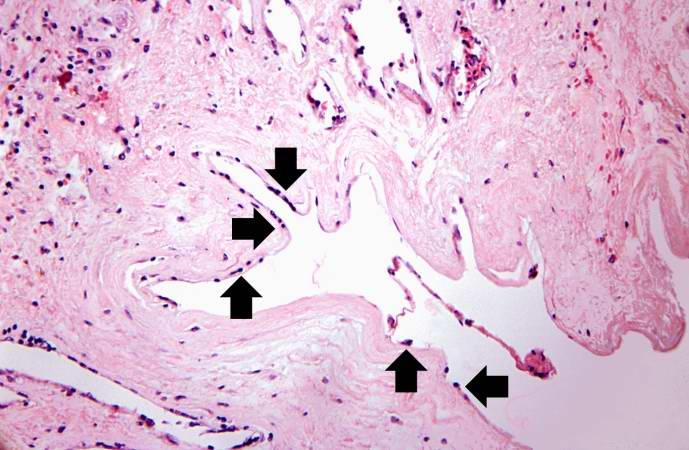
Image:Thromboembolus 5.jpg|This is a photomicrograph of the wall of the pulmonary artery (1) containing the thromboembolus. In this case the artery wall looks normal. If this was a thrombus instead of a thromboembolus, you would expect to see some damage in the artery wall that would have initiated the thrombus. Note the lines of Zahn in the thromboembolus (arrows). | |||
Image:Thromboembolus 6.jpg|This is a low-power photomicrograph of the infarcted lung. The tissue is congested and has a very bland appearance due to coagulation necrosis of the lung parenchyma. You can still see the outlines of the alveoli and the cells that make-up the alveoli but there is almost complete loss of nuclei throughout this section. | [[Image:Thromboembolus 4.jpg|left|thumb|400px|This is a low-power photomicrograph of lung. A large thrombus is lodged at this branch point in the pulmonary artery. Note the hemorrhage and congestion in the surrounding lung parenchyma.]] | ||
</ | <br clear="left"/> | ||
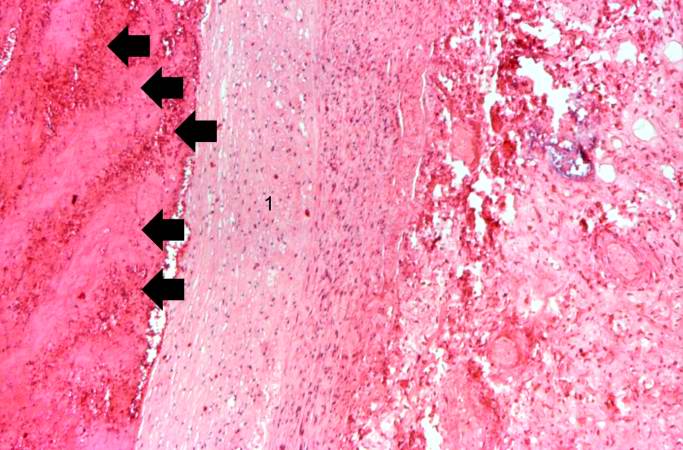
[[Image:Thromboembolus 5.jpg|left|thumb|400px|This is a photomicrograph of the wall of the pulmonary artery (1) containing the thromboembolus. In this case the artery wall looks normal. If this was a thrombus instead of a thromboembolus, you would expect to see some damage in the artery wall that would have initiated the thrombus. Note the lines of Zahn in the thromboembolus (arrows).]] | |||
<br clear="left"/> | |||
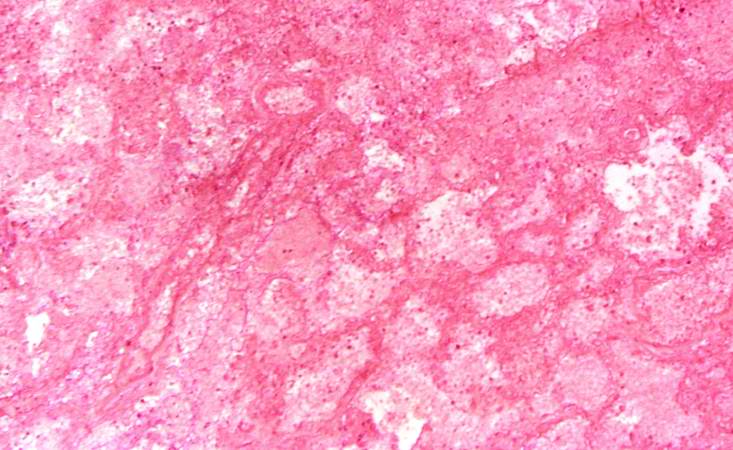
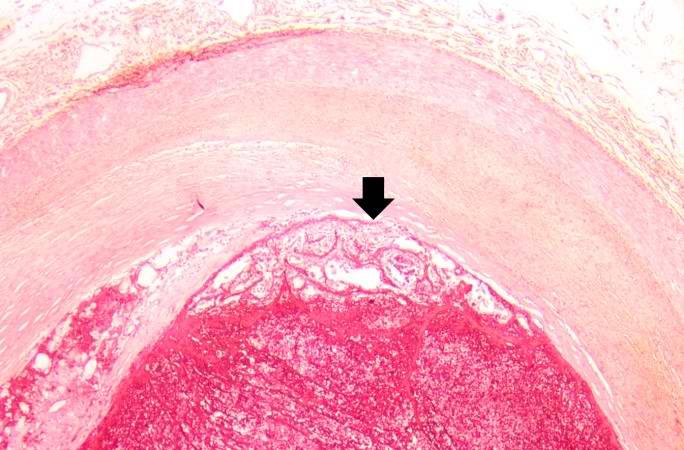
[[Image:Thromboembolus 6.jpg|left|thumb|400px|This is a low-power photomicrograph of the infarcted lung. The tissue is congested and has a very bland appearance due to coagulation necrosis of the lung parenchyma. You can still see the outlines of the alveoli and the cells that make-up the alveoli but there is almost complete loss of nuclei throughout this section.]] | |||
<br clear="left"/> | |||
===Thromboembolism: Testes=== | ===Thromboembolism: Testes=== | ||
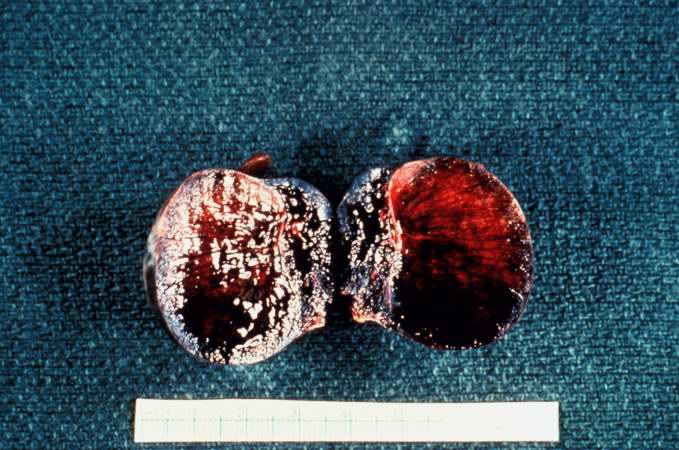
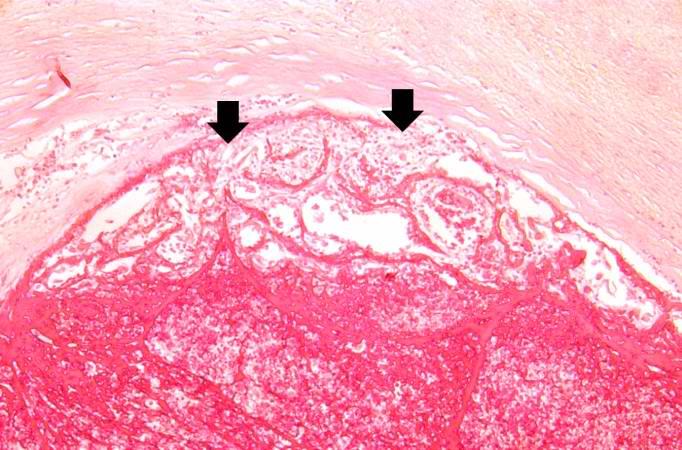
[[Image:Thromboembolus 7.jpg|left|thumb|400px|This is a gross photograph of an infarcted testis. Because of the anatomy of the blood supply to the testis, torsion or the blood vessels often leads to venous occlusion (due to compression of the thin walled veins) but not arterial occlusion. Thus, blood still flows into the testis but it can’t get out! This leads to hypoxia and eventually to hemorrhagic necrosis. ]] | |||
Image:Thromboembolus 7.jpg|This is a gross photograph of an infarcted testis. Because of the anatomy of the blood supply to the testis, torsion or the blood vessels often leads to venous occlusion (due to compression of the thin walled veins) but not arterial occlusion. Thus, blood still flows into the testis but it can’t get out! This leads to hypoxia and eventually to hemorrhagic necrosis. | <br clear="left"/> | ||
Image:Thromboembolus 8.jpg|This is a gross photograph of cut section of testis from previous image. The tissue is filled with blood. | |||
</ | [[Image:Thromboembolus 8.jpg|left|thumb|400px|This is a gross photograph of cut section of testis from previous image. The tissue is filled with blood. ]] | ||
<br clear="left"/> | |||
===Thromboembolism: Bowel infarction=== | ===Thromboembolism: Bowel infarction=== | ||
Revision as of 02:22, 26 February 2009
| Thromboembolism |
|
WikiDoc Resources for Thromboembolism |
|
Articles |
|---|
|
Most recent articles on Thromboembolism Most cited articles on Thromboembolism |
|
Media |
|
Powerpoint slides on Thromboembolism |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Thromboembolism at Clinical Trials.gov Trial results on Thromboembolism Clinical Trials on Thromboembolism at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Thromboembolism NICE Guidance on Thromboembolism
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Thromboembolism Discussion groups on Thromboembolism Patient Handouts on Thromboembolism Directions to Hospitals Treating Thromboembolism Risk calculators and risk factors for Thromboembolism
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Thromboembolism |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Thromboembolism is a general term describing both thrombosis and its main complication which is embolisation.
Differential Diagnosis of Causes of Thromboembolism
- Acute lymphoblastic leukemia
- Acute myelocytic leukemia
- Antiphospholipid antibody syndrome
- Atrial fibrillation
- Bacterial meningitis
- Baker’s cyst
- Central nervous system tumors
- Congenital heart disease
- Consumption coagulopathy
- Head trauma
- Hypertrophic cardiomyopathy
- Nephrotic syndrome
- Pneumonia
- Sepsis
- Thrombophlebitis
- Trauma
- Vasculitis
Etymology
The term was coined in 1848 by Rudolph Carl Virchow.[1]
Incidence
In the United States:
- 300,000–600,000 people have deep venous thrombosis (DVT) or pulmonary embolism (PE).
- 200,000–400,000 people have deep venous thrombosis.
- Nearly one-third of people who have had deep venous thrombosis have post-thrombotic syndrome, a chronic disabling condition characterized by swelling, pain, discoloration, and scaling in the affected limb.
- 100,000–200,000 people have a pulmonary embolism.
- Nearly one-third of people (30,000–60,000) who have a pulmonary embolism die.
- 5-8% of people have thrombophilia (inherited blood clotting disorders).
Demographics
Pathophysiology
The formation of a thrombus is usually caused by the top three causes, known as (Virchow's triad): (Classically, thrombosis is caused by abnormalities in one or more of the following)
- The composition of the blood (hypercoagulability)
- Quality of the vessel wall (endothelial cell injury)
- Nature of the blood flow (hemostasis)
To elaborate, the pathogenesis includes:
- an injury to the vessel's wall (such as by trauma, infection, or turbulent flow at bifurcations);
- by the slowing or stagnation of blood flow past the point of injury (which may occur after long periods of sedentary behavior (for example, sitting on a long airplane flight);
- by a blood state of hypercoagulability (caused for example, by genetic deficiencies or autoimmune disorders).
High altitude has also been known to induce thrombosis [2] [3]. Occasionally, abnormalities in coagulation are to blame. Intravascular coagulation follows, forming a structureless mass of red blood cells, leukocytes, and fibrin.
Classification
A. Thrombosis
There are two distinct forms of thrombosis:
Venous thrombosis
- Deep venous thrombosis (with or without pulmonary embolism; together classified as venous thromboembolism/VTE)
- Portal vein thrombosis
- Renal vein thrombosis
- hepatic vein thrombosis (Budd-Chiari syndrome)
- Paget-Schroetter disease
- Cerebral venous sinus thrombosis
- Thoracic outlet syndrome (the cause of most Subclavian vein thrombosis unrelated to trauma)
Arterial thrombosis
B. Embolism
If a bacterial infection is present at the site of thrombosis, the thrombus may break down, spreading particles of infected material throughout the circulatory system (pyemia, septic embolus) and setting up metastatic abscesses wherever they come to rest.
Without an infection, the thrombus may become detached and enter circulation as an embolus, finally lodging in and completely obstructing a blood vessel (an infarction). The effects of an infarction depend on where it occurs.
The pathway of the embolism can be one of three types:
- Anterograde
- Retrograde
- Paradoxical
In anterograde embolism, the movement of emboli is in the direction of blood flow. In retrograde embolism, however, the emboli move in opposition to the blood flow direction; this is usually significant only in blood vessels with low pressure (veins) or with emboli of high weight. In paradoxical embolism, also known as crossed embolism, an embolus from the veins crosses to the arterial blood system. This is generally found only with heart problems such as septal defects between the atria or ventricles.
Sources of Systemic Embolism
- Left ventricular thrombus
- Left atrial thrombus
- Pelvic veins or lower extremity thrombus
- Native cardiac valves
- Prosthetic cardiac valves
- Cardiac tumors
- Aortic aneurysm
- Invasive manipulations
- Left ventricular aneurysm
-
Sources of Systemic Embolism: Large left ventricular thrombus in a patient with myocardial infarction
-
Sources of Systemic Embolism: Large left ventricular thrombus in a patient with myocardial infarction
Risk Factors
Almost anyone can have thromboembolic event. However, certain factors can increase the risk of developing this condition. The risk increases even more for someone who has more than one risk factor at the same time.
- Sedentary life style
- Elderly
- Atrial fibrillation
- Sepsis
- Prolonged bed rest and / or immobility
- Pregnancy
- Hyperemesis
- Dehydration
- Cesarean delivery
- Congestive heart failure
- Nephrotic syndrome
- Severe varicose veins
- Family history of thromboembolism
- Thrombophilia
- Factor V Leiden mutation
- Prothrombin G20210A mutation
- Decreased antithrombin level
- Decreased Protein C level
- Decreased Protein S level
- Antiphospholipid syndrome
- Long air travel
- Oral contraceptives
- Obesity
- Smoking
- Minor injuries[4]
Following is a list of factors that increase the risk of developing deep vein thrombosis
- Injury to the vein, often caused by:
- Fractures,
- Severe muscle injury,
- Major surgery (particularly involving the abdomen, pelvis, hip, or legs).
- Slow blood flow, often caused by:
- Confinement to bed (e.g., due to a medical condition or after surgery);
- Limited movement (e.g., a cast on a leg to help heal an injured bone;
- Sitting for a long time, especially with crossed legs; or
- Paralysis.
- Increased estrogen, often caused by:
- Birth control pills;
- Hormone replacement therapy, sometimes used after menopause; or
- Pregnancy, for up to 6 weeks after giving birth.
- Certain chronic medical illnesses, such as:
- Trauma
- Multiple trauma
- CNS/spinal cord injury
- Burns
- Lower extremity fractures
- Other risk factors include:
- Previous DVT
- Family history of DVT
- Age (risk increases as age increases)
- Obesity
- Smoking
- High blood pressure
- A catheter located in a central vein
- Inherited clotting disorders. An inherited clotting disorder might be suspected when a person has repeated DVTs that cannot be linked to any specific cause (such as recent surgery) or develops DVT in a vein at an unusual location, such as a vein in the liver, kidney, or brain.
Diagnosis
Diagnostic modalities may differ for deep venous thrombosis and pulmonary embolism. Some patients may have the both clinical situations.
History and Symptoms
There are several techniques during physical examination to increase the detection of DVT, such as measuring the circumference of the affected and the contralateral limb at a fixed point (to objectivate edema), and palpating the venous tract, which is often tender. Physical examination alone is unreliable for excluding the diagnosis of deep vein thrombosis.
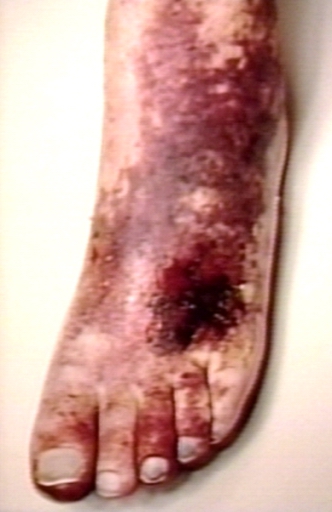
In phlegmasia alba dolens, the leg is pale and cool with a diminished arterial pulse due to spasm. It usually results from acute occlusion of the iliac and femoral veins due to DVT.
In phlegmasia cerulea dolens, there is an acute and nearly total venous occlusion of the entire extremity outflow, including the iliac and femoral veins. The leg is usually painful, cyanosed and oedematous. Venous gangrene may supervene.
It is vital that the possibility of pulmonary embolism be included in the history, as this may warrant further investigation (see pulmonary embolism).
A careful history has to be taken considering risk factors of thromboembolism, including the use of estrogen-containing methods of hormonal contraception, recent long-haul flying, and a history of miscarriage (which is a feature of several disorders that can also cause thrombosis). A family history can reveal a hereditary factor in the development of DVT.
The symptoms and signs of thromboembolism may include:
- Tachypnea
- Dyspnea
- Chest pain
- Discomfort in the legs
- Pleuritic pain
- Apprehension and anxiety
- Cough
- Tachycardia
- Syncope
- Right heart failure
- Jugular venous distention
- Hepatomegaly
- Left parasternal heave
- Fixed splitting of the second heart sound
- Dilation of the surface veins
- Hemoptysis
- Fever
- Hyperemia in thrombosis, pallor in embolism
- Local swelling
- Local pain
- Tenderness
- Very low blood pressure
- Lightheadedness
Physical Examination
- Homans' sign
- Pratt's sign: Squeezing of posterior calf elicits pain.
However, these medical signs do not perform well and are not included in clinical prediction rules that combine best findings in order to diagnose DVT.[5]
(Images courtesy of Charlie Goldberg, M.D., UCSD School of Medicine and VA Medical Center, San Diego, California)
-
Deep venous thrombosis: Right Lower Extremity DVT
-
Deep venous thrombosis: Left Lower Extremity DVT
-
Deep venous thrombosis: Left Lower Extremity DVT
Image:upper dvt.jpg|Deep venous thrombosis: Diffusely swollen RUE resulting from a PICC line induced thrombosis. Image:extremities_dvt4.jpg|Left Lower Extremity DVT:Note diffusely swollen left leg. skin changes on left are due to chronic venous insufficiency. </gallery>
-
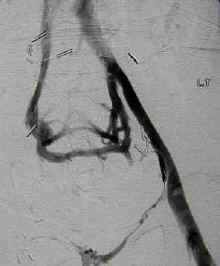
Thromboembolic event of popliteal artery
-
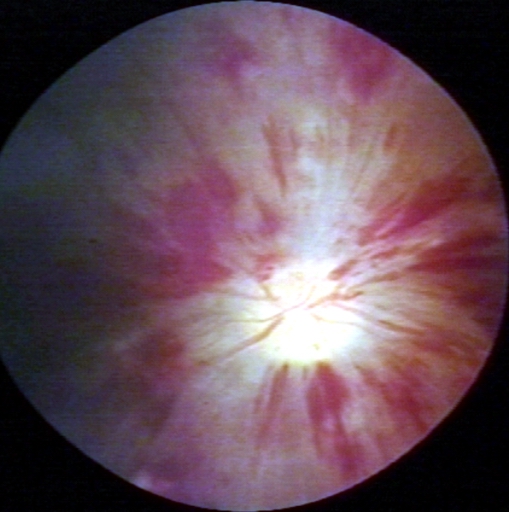
Fundoscopy: Central retinal vein thrombosis
Probability scoring
In 2006, Scarvelis and Wells overviewed a set of clinical prediction rules for DVT,[6] on the heels of a widely adopted set of clinical criteria for pulmonary embolism.[7] [8]
Wells score or criteria
(Possible score -2 to 9)
- 1) Active cancer (treatment within last 6 months or palliative) -- 1 point
- 2) Calf swelling >3 cm compared to other calf (measured 10 cm below tibial tuberosity) -- 1 point
- 3) Collateral superficial veins (non-varicose) -- 1 point
- 4) Pitting edema (confined to symptomatic leg) -- 1 point
- 5) Swelling of entire leg - 1 point
- 6) Localized pain along distribution of deep venous system -- 1 point
- 7) Paralysis, paresis, or recent cast immobilization of lower extremities -- 1 point
- 8) Recently bedridden > 3 days, or major surgery requiring regional or general anesthetic in past 12 weeks -- 1 point
- 9) Previously documented DVT -- 1 point
- 10) Alternative diagnosis at least as likely -- Subtract 2 points
Interpretation
Traditional interpretation [8] [9]
- Score >6.0 - High (probability 59% based on pooled data [10])
- Score 2.0 to 6.0 - Moderate (probability 29% based on pooled data[10])
- Score <2.0 - Low (probability 15% based on pooled data[10])
Alternate interpretation
- Score > 4 - PE likely. Consider diagnostic imaging.[8] [11]
- Score 4 or less - PE unlikely. Consider D-dimer to rule out PE.
Laboratory Tests
In low/moderate suspicion of PE, a normal D-dimer level (shown in a blood test) is enough to exclude the possibility of thrombotic PE.[12]
When a PE is being suspected, a number of blood tests are done, in order to exclude important secondary causes of PE. This includes a full blood count, clotting status (PT, APTT, TT), and some screening tests (erythrocyte sedimentation rate, renal function, liver enzymes, electrolytes). If one of these is abnormal, further investigations might be warranted.
Plasma D-dimer level: D-dimer is a fibrin degradation product and an important marker of activated fibrinolysis. Enzyme linked immunoassay and latex turbidimetric assays methods provide its quantity. It can be elevated in pneumonia, cancer, sepsis, and after surgery. D-dimer values increase progressively throughout pregnancy, and the ranges for normal values by gestational week are not yet universally established. With low or moderate clinical suspicion, a negative d-dimer test rules out pulmonary embolism.
Arterial Blood Gas
- Acid-base status may demonstrate a respiratory alkalosis.
- The arterial blood gas in room temperature demonstrates hypoxemia (PaO2 <80 mm Hg) and an elevated alveolar / arterial oxygen gradient.
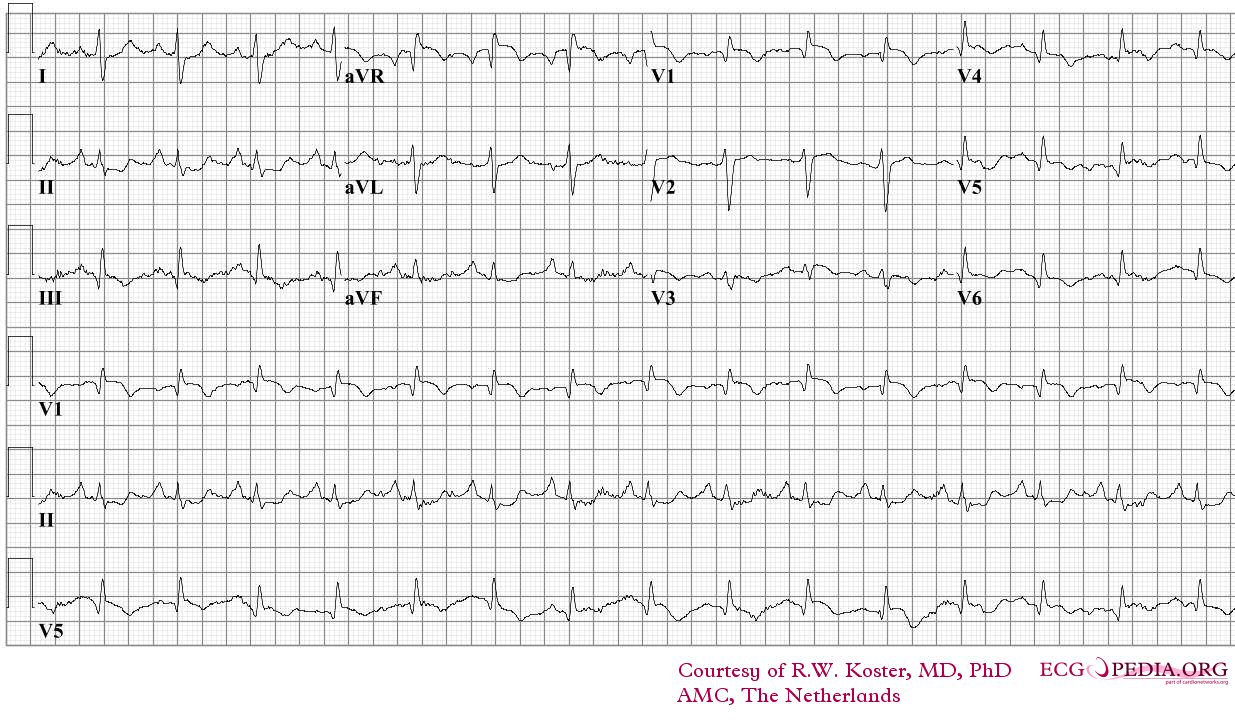
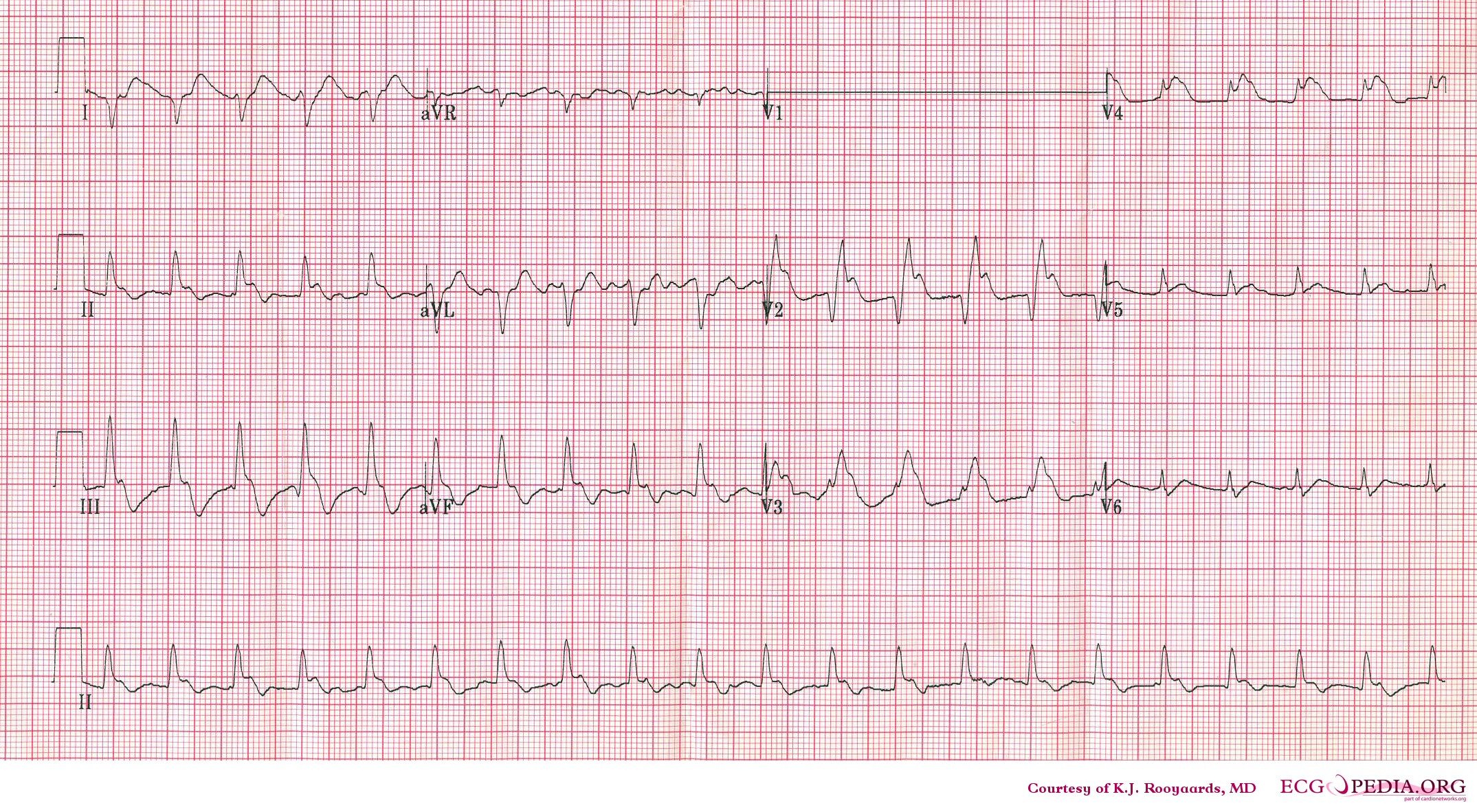
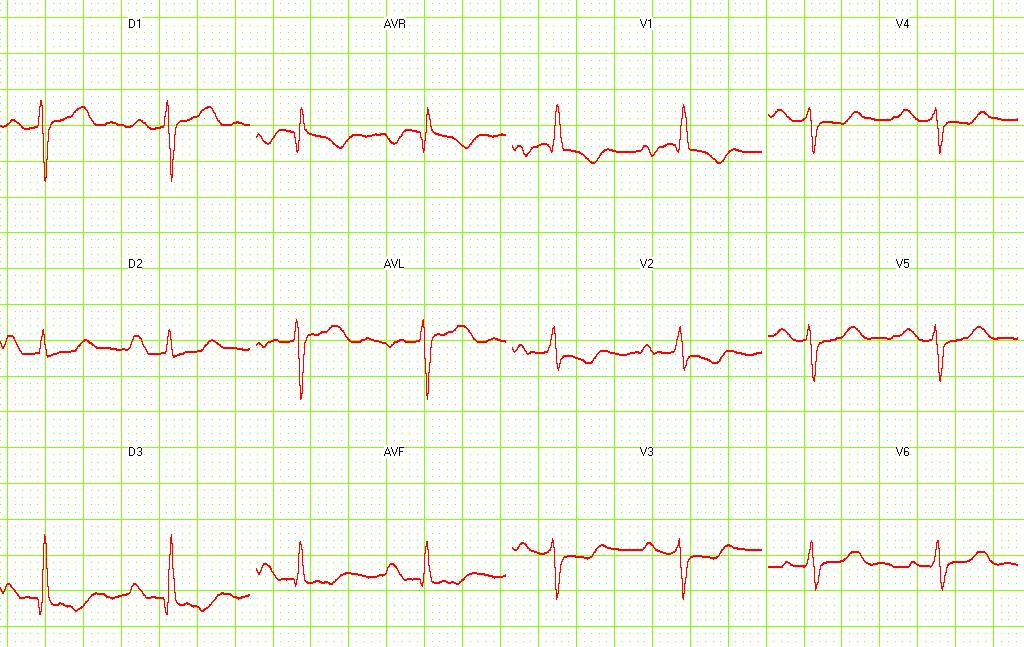
Electrocardiography
- Sinus tachycardia
- Right axis deviation
- Right bundle branch block
- Deep and inverted T waves in V1 - V3
- S1Q3T3 pattern
-
ECG of a patient with pulmonary embolism Image courtesy of ecgpedia
-
Another example; a patient with pulmonary embolism. Note the tachycardia and right axis.Image courtesy of ecgpedia
-
Pulmonary embolism. S1-Q3 and signs of right frontal axis are shown. Image courtesy of Dr Jose Ganseman Dr Ganseman's webpage
X-ray
- In normal range in majority of cases
- Chest x rays may reveal an enlarged right descending pulmonary artery
- Decreased pulmonary vascularity (Westermark sign)
- A wedge shaped infiltrate
- An elevation of the hemidiaphragm (Hampton's hump)
- Pleural effusion (usually predicts the presence of an infarction)
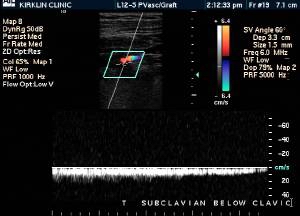
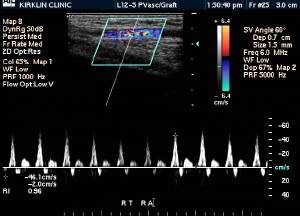
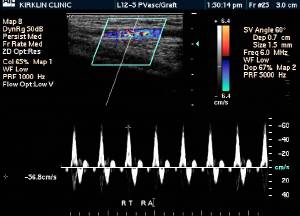
Doppler Ultrasonography
-
Deep venous thrombosis
-
Deep venous thrombosis
-
Deep venous thrombosis
-
Deep venous thrombosis
(Images shown below are courtesy of RadsWiki)
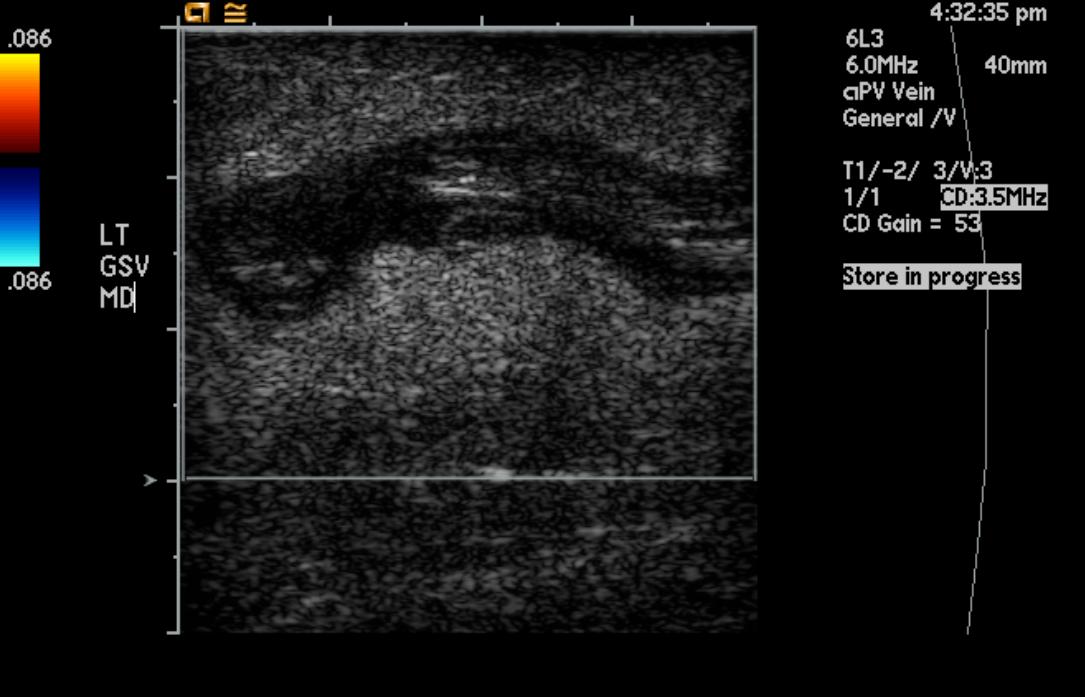
-
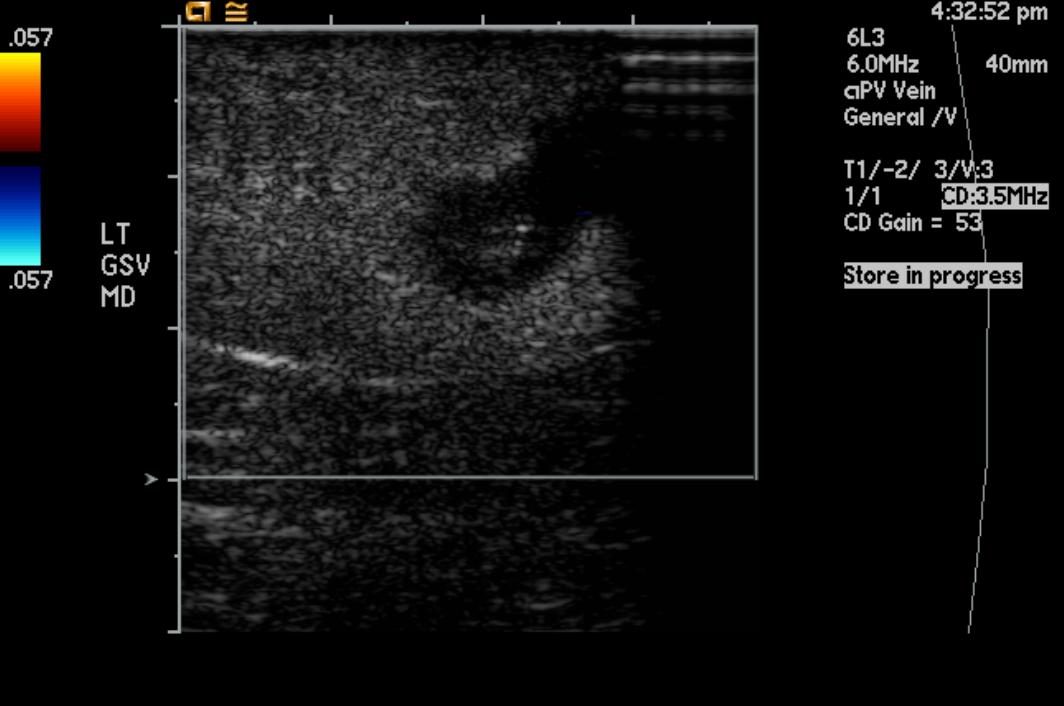
Greater saphenous vein thrombosis
-
Greater saphenous vein thrombosis
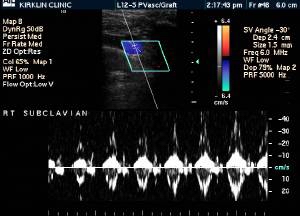
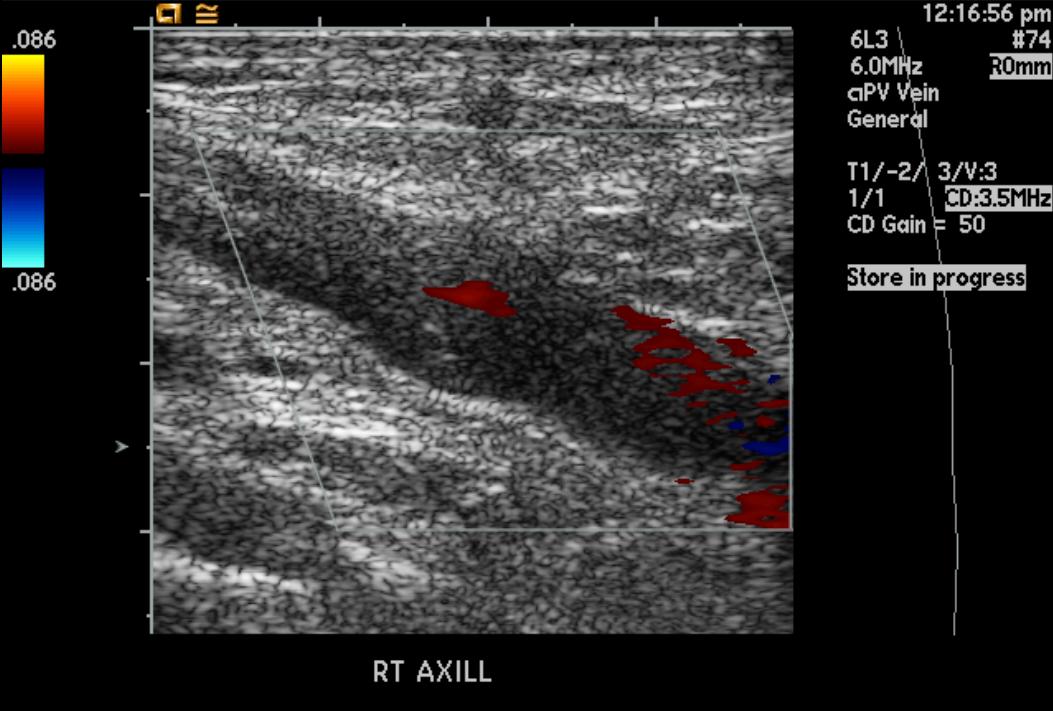
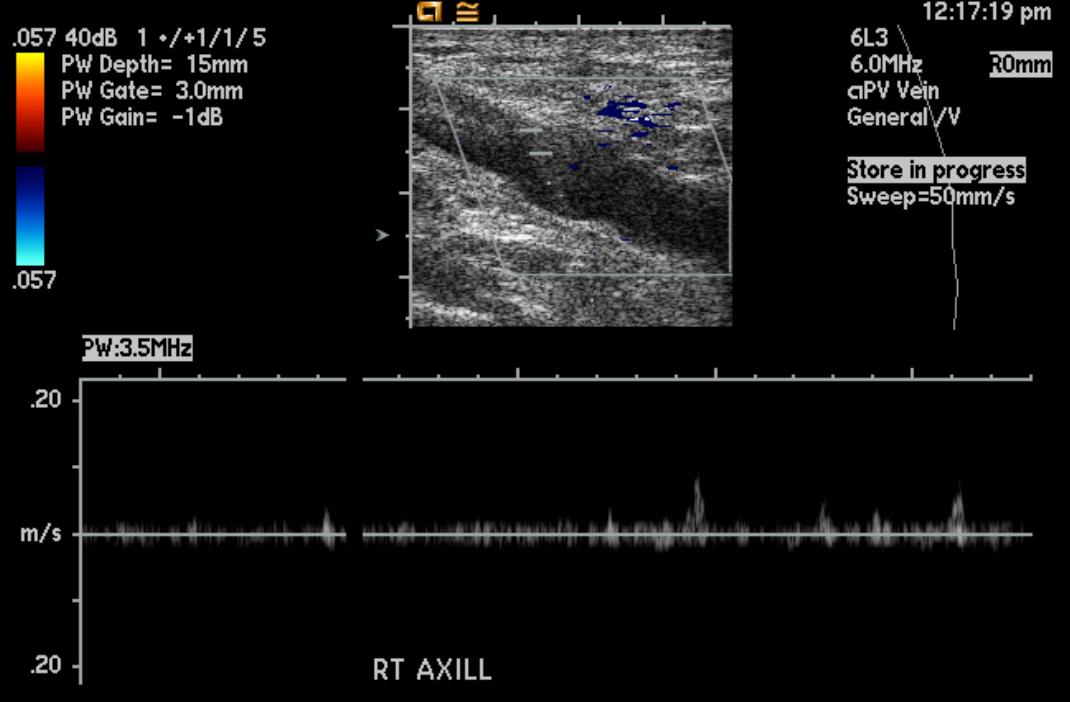
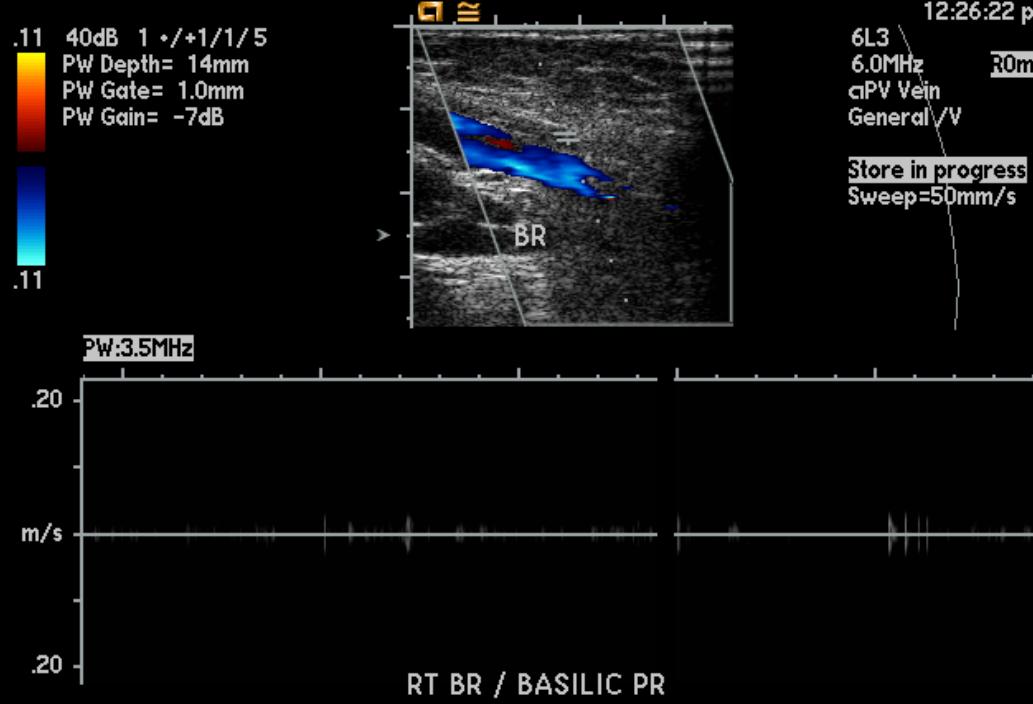
-
Upper extremity deep vein thrombosis
-
Upper extremity deep vein thrombosis
-
Upper extremity deep vein thrombosis
-
Upper extremity deep vein thrombosis
Echocardiography
- Thrombus in Left Atrial Appendage
<googlevideo>1194621255843080155&hl=en</googlevideo>
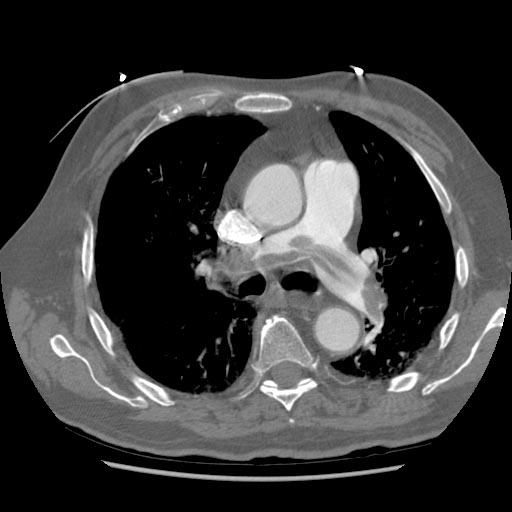
Computed Tomography and CT Angiography
Computed tomography with radiocontrast, effectively a pulmonary angiogram imaged by CT and also known as CT pulmonary angiography (CTPA), is increasingly used as the mainstay in diagnosis. Advantages are clinical equivalence, its non-invasive nature, its greater availability to patients, and the possibility of picking up other lung disorders from the differential diagnosis in case there is no pulmonary embolism.
CT findings in Acute PE
- Thrombus is located centrally within the vascular lumen or occludes the vessel (vessel cut-off sign)
- Commonly causes distention of the involved vessel.
CT findings in Chronic PE
- Eccentric and contiguous changes of the vessel wall
- Reduces the arterial diameter by more than 50%
- Evidence of recanalization within the thrombus
- An arterial web is present
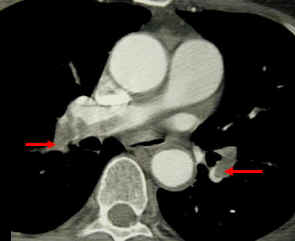
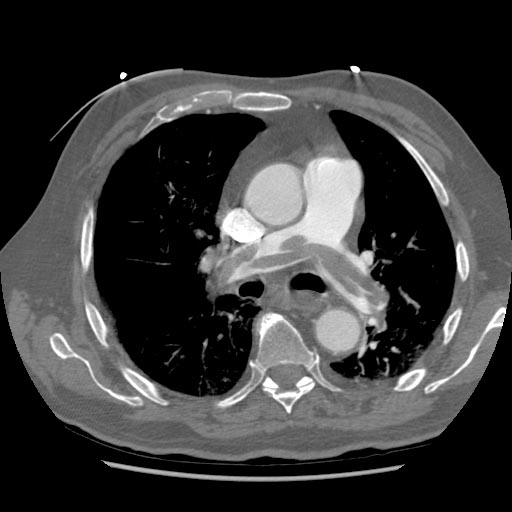
-
Left leg. Deep venous thrombosis
-
Left leg. Deep venous thrombosis
-
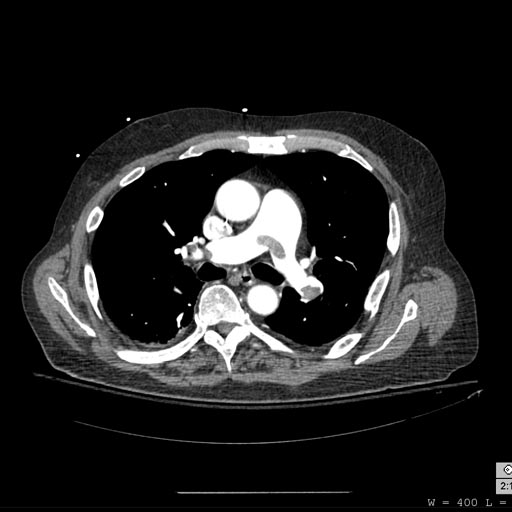
CT pulmonary angiogram. Clots in both the left and the right pulmonary arteries (red arrows). Source
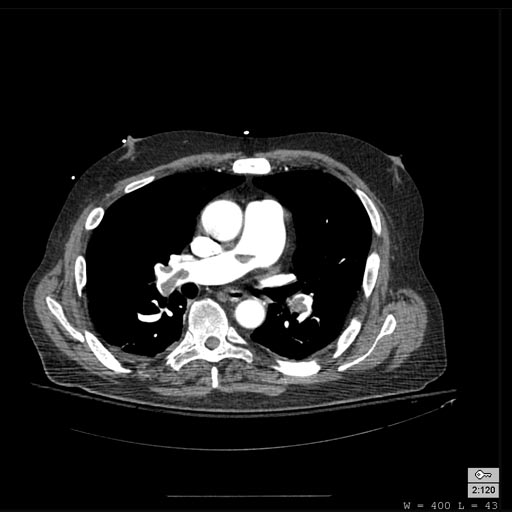
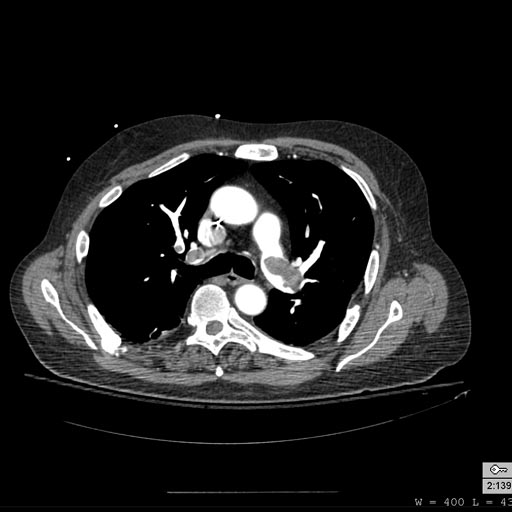
Patient with Shortness of Breath
-
Pulmonary embolism: Patient presented with Shortness of breath
-
Pulmonary embolism: Patient presented with Shortness of breath
-
Pulmonary embolism: Patient presented with Shortness of breath
-
Pulmonary embolism: Patient presented with Shortness of breath
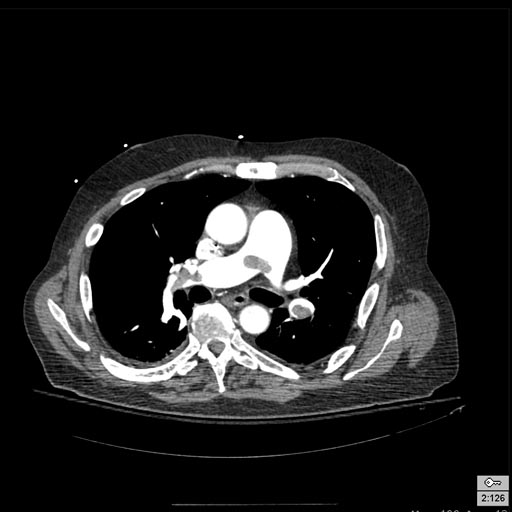
Patient with Acute RBBB
-
Pulmonary embolism: Patient presented with Acute RBBB
-
Pulmonary embolism: Patient presented with Acute RBBB
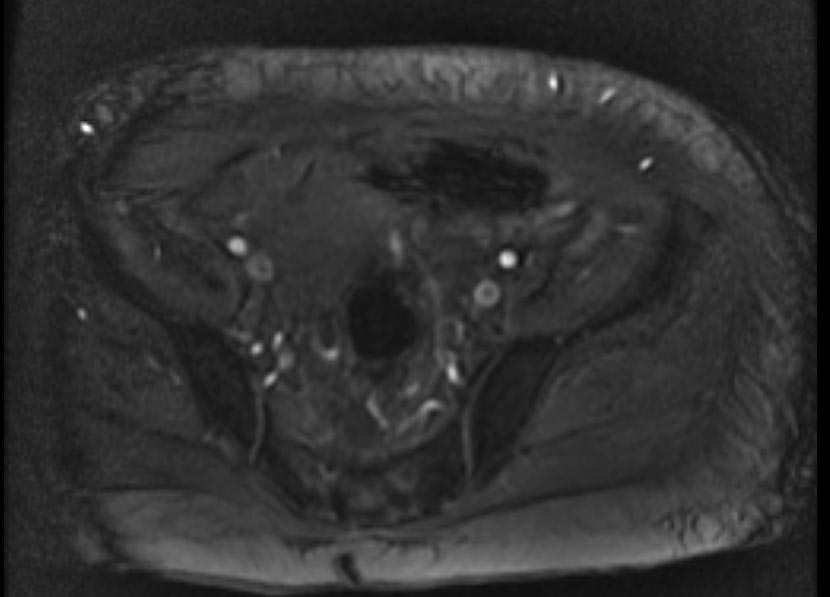
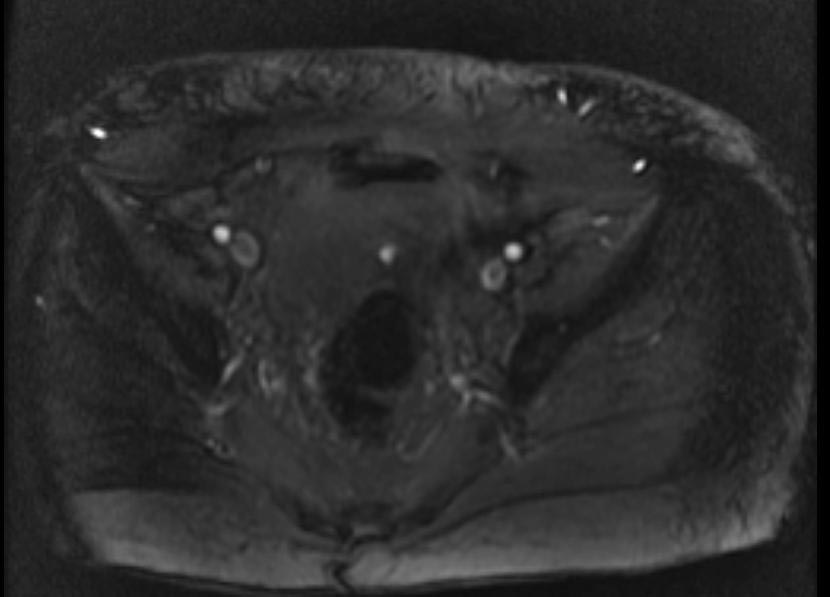
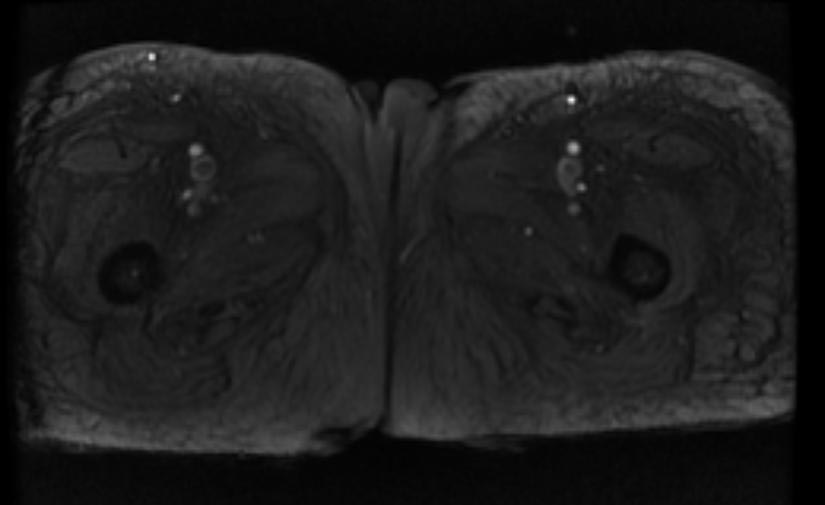
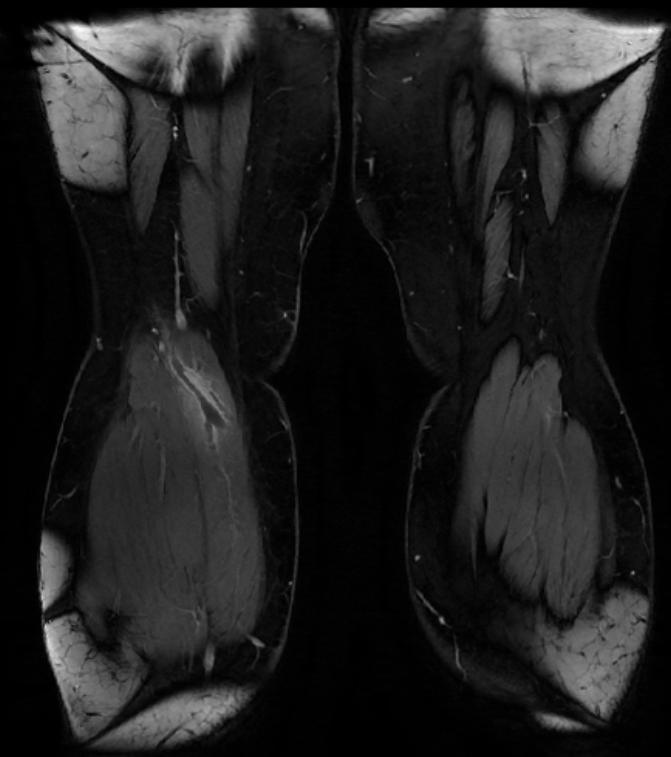
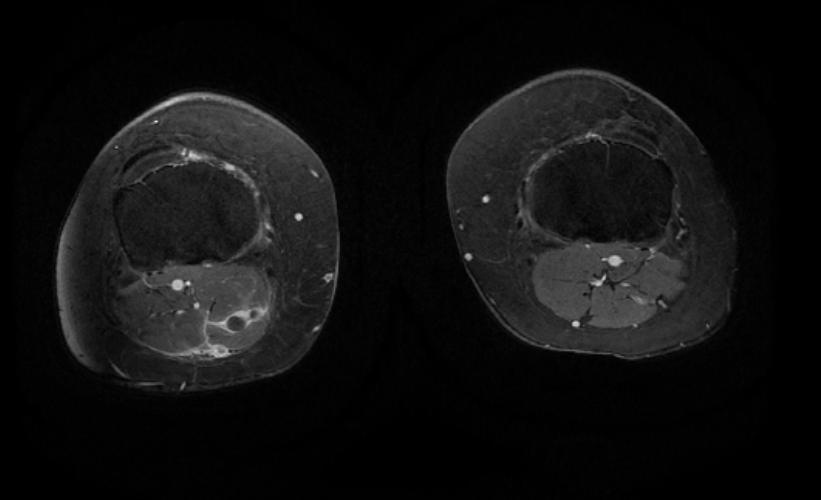
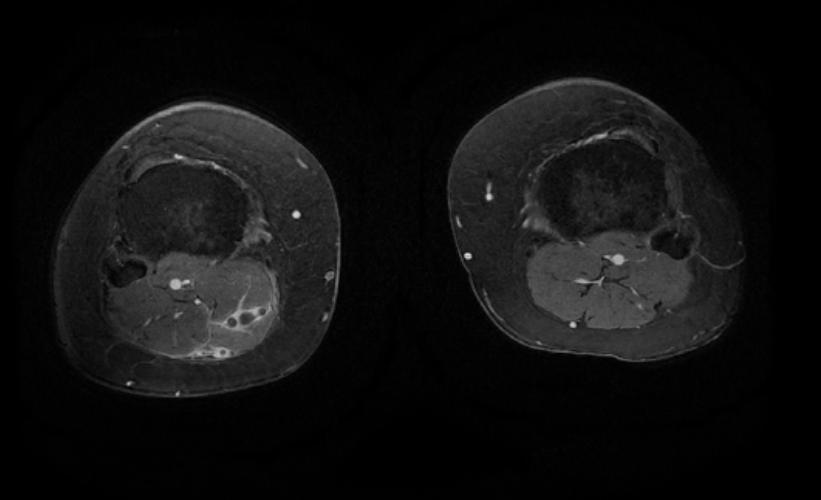
MR and MR Angiography
- Gadolinium-enhanced MRI is a non-invasive diagnostic modality and has the advantage of no contrast exposure.
- A potential benefit of MR, is that is incredibly sensitive, perhaps even better than contrast venography, in imaging clot in the inferior vena cava (IVC) and pelvic veins, and these images can be obtained at the same time as the lung scan.
- It needs to be pointed out, that although the criticism of using CT and MR angio lacks sensitivity when examining the subsegmental arteries, inter-reader agreement was only 66% with pulmonary angiography in PIOPED.
{Images shown below are courtesy of RadsWiki)
-
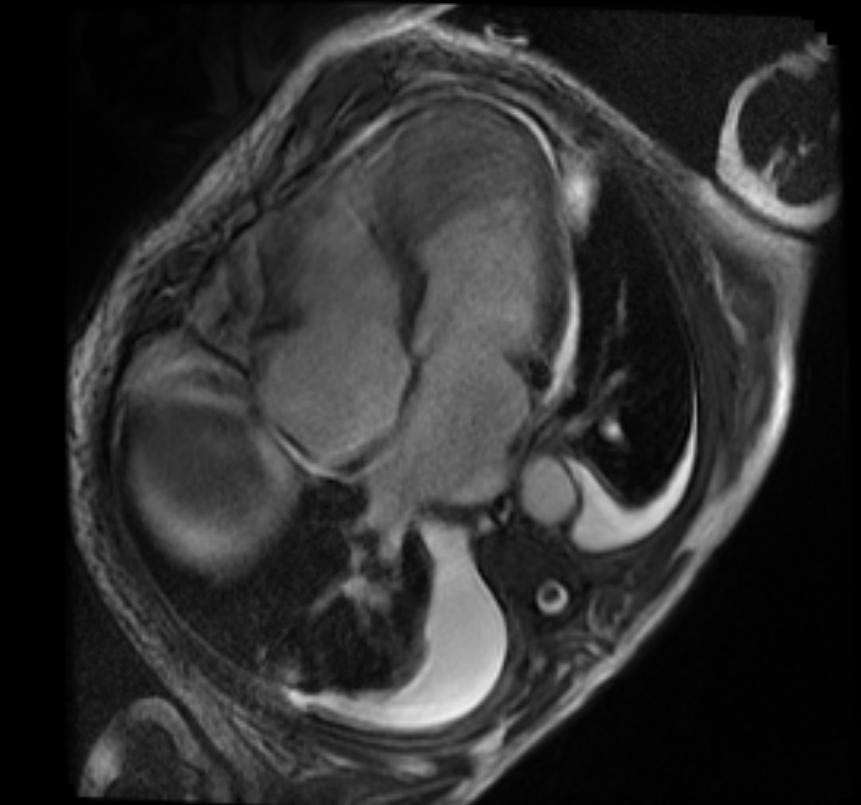
2D TOF GRE MRV images: Bilateral deep vein thrombosis
-
2D TOF GRE MRV images: Bilateral deep vein thrombosis
-
2D TOF GRE MRV images: Bilateral deep vein thrombosis
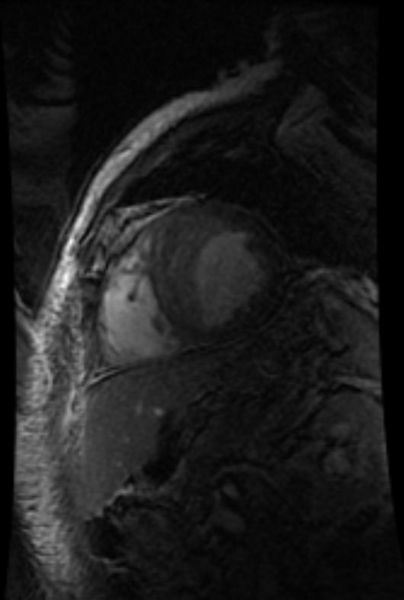
-
MRI: Superficial vein thrombosis
-
MRI: Superficial vein thrombosis
-
MRI: Superficial vein thrombosis
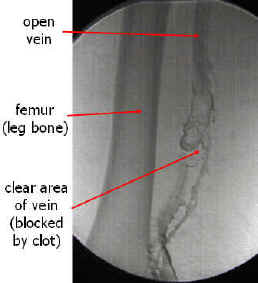
Contrast Venography
Contrast venography (also called Venography or phlebography) is the definitive test for diagnosing deep venous thrombosis which taken after a special dye is injected into the vein or even bone marrow.
Contrast venography can also help;
- to distinguish blood clots from obstructions in the veins
- to evaluate congenital vein problems
- to evaluate veins prior to treatment of chronic venous insufficiency
- to control functioning of deep leg vein valves
- to identify a vein graft for coronary artery bypass surgery
-
Venography: Deep venous thrombosis. Source
-
An occluded vein with collateral vessel formation. Source
Pulmonary Angiography
Pulmonary angiography (or pulmonary arteriography) is a cardiological medical procedure. Pulmonary arteries are visualized to detect blood clots (such as a pulmonary embolism) or arteriovenous malformations.
The use of pulmonary angiography has been largely replaced by spiral CT in diagnosis of pulmonary embolism.
-
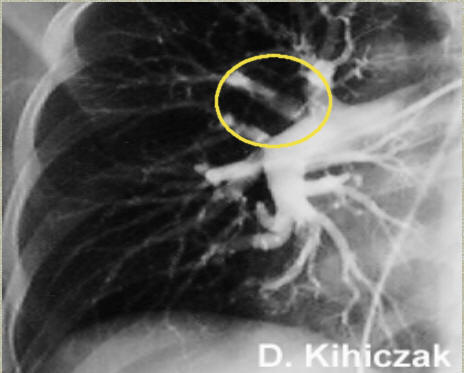
Pulmonary angiogram in a patient with pulmonary embolus. A thrombus is observed in the area within the yellow circle. Source
Ventilation / Perfusion Scan
Ventilation/perfusion scan (or V/Q scan or lung scintigraphy), which shows that some areas of the lung are being ventilated but not perfused with blood (due to obstruction by a clot). This type of examination is used less often because of the more widespread availability of CT technology, however, it may be useful in patients who have an allergy to iodinated contrast or in pregnancy due to lower radiation exposure than CT. * The ventilation/perfusion ratio (V/Q) Scan: The PIOPED data suggests that normal perfusion scans are almost never associated with recurrent pulmonary embolism, even if anticoagulation is withheld.
Other Methods
Impedance plethysmography
Impedance phlebography or impedance plethysmography is a non-invasive medical test that measures small changes in electrical resistance of the chest, calf or other regions of the body. These measurements reflect blood volume changes, and can indirectly indicate the presence or absence of venous thrombosis. This procedure provides an alternative to venography, which is invasive and requires a great deal of skill to execute adequately and interpret accurately.
For the chest, the technique was developed by NASA to measure the split second impedance changes within the chest, as the heart beats, to calculate both cardiac output and lung water content. This technique has progressed clinically (often now called BioZ, i.e. biologic impedance) and allows low cost, non-invasive estimations of cardiac output and total peripheral resistance, using only 4 skin electrodes, oscillometric blood pressure measurement and lung water volumes with minimal removal of clothing in physician offices having the needed equipment.
For leg veins, the test measures blood volume in the lower leg due to temporary venous obstruction. This is accomplished by inflating a pneumatic cuff around the thigh to sufficient pressure to cut off venous flow but not arterial flow, causing the venous blood pressure to rise until it equals the pressure under the cuff. When the cuff is released there is a rapid venous runoff and a prompt return to the resting blood volume. Venous thrombosis will alter the normal response to temporary venous obstruction in a highly characteristic way, causing a delay in emptying of the venous system after the release of the tourniquet. The increase in blood volume after cuff inflation is also usually diminished.
Treatment
A. Deep Venous Thrombosis
Hospitalization
Treatment at home is an option according to a meta-analysis by the Cochrane Collaboration.[13]
Hospitalization should be considered in patients with more than two of the following risk factors as these patients may have more risk of complications during treatment[14]:
- bilateral deep venous thrombosis
- renal insufficiency
- body weight <70 kg
- recent prolonged immobility
- chronic heart failure
- cancer
Anticoagulation
Anticoagulation is the usual treatment for DVT. In general, patients are initiated on a brief course (i.e., less than a week) of heparin treatment while they start on a 3- to 6-month course of warfarin (or related vitamin K inhibitors). Low molecular weight heparin (LMWH) is preferred,[15] though unfractionated heparin is given in patients who have a contraindication to LMWH (e.g., renal failure or imminent need for invasive procedure). In patients who have had recurrent DVTs (two or more), anticoagulation is generally "life-long." The Cochrane Collaboration has meta-analyzed the risk and benefits of prolonged anti-coagulation.[16]
An abnormal D-dimer level at the end of treatment might signal the need for continued treatment among patients with a first unprovoked proximal deep-vein thrombosis.[17]
Thrombolysis
Thrombolysis is generally reserved for extensive clot, e.g. an iliofemoral thrombosis. Although a meta-analysis of randomized controlled trials by the Cochrane Collaboration shows improved outcomes with thrombolysis,[18] there may be an increase in serious bleeding complications.
Inferior vena cava filter
Inferior vena cava filter reduces pulmonary embolism[19] and is an option for patients with an absolute contraindiciation to anticoagulant treatment (e.g., cerebral hemorrhage) or those rare patients who have objectively documented recurrent PEs while on anticoagulation, an inferior vena cava filter (also referred to as a Greenfield filter) may prevent pulmonary embolisation of the leg clot. However these filters are themselves potential foci of thrombosis,[20] IVC filters are viewed as a temporizing measure for preventing life-threatening pulmonary embolism.[21]
Compression stockings
Elastic compression stockings should be routinely applied "beginning within 1 month of diagnosis of proximal DVT and continuing for a minimum of 1 year after diagnosis".[15] Starting within one week may be more effective.[22] The stockings in almost all trials were stronger than routine anti-embolism stockings and created either 20-30 mm Hg or 30-40 mm Hg. Most trials used knee-high stockings. A meta-analysis of randomized controlled trials by the Cochrane Collaboration showed reduced incidence of post-phlebitic syndrome.[23] The number needed to treat is quite potent at 4 to 5 patients need to prevent one case of post-phlebitic syndrome.[24]
Surgical Therapy
- Thrombectomy
- Venous ligation: Not used anymore
B. Pulmonary Embolism
Emergency treatment at a hospital is necessary to treat pulmonary embolism.
Acute Pharmacotherapy
Chronic Pharmacotherapy
Surgical Therapy
- Pulmonary embolectomy: it has a high mortality rate.
Treatment in Special Population
1. Pregnancy
- Warfarin is contraindicated during pregnancy. It crosses the placenta and increases the risk of miscarriage, stillbirth, embryopathy (nasal hypoplasia or stippled epiphyses), central nervous system abnormalities, maternal hemorrhage and fetal hemorrhage. It is safe to use it in postpartum period and is compatible with breastfeeding.
- Low-molecular-weight heparin has largely replaced unfractionated heparin for prophylaxis and treatment.
2. Elderly
3. Renal Failure
4. Newborn and Early Childhood
American Heart Association's Guidelines
American Family Physician's Guidelines
Complications
- Postthrombotic syndrome
- Chronic thromboembolic pulmonary hypertension
Prevention
Pathological Findings
- A 67-year-old male was hospitalized because of extensive atherosclerotic cardiovascular disease. Following surgery, during which diseased portions of the femoral arteries were bypassed, he developed massive pulmonary embolism and expired. At autopsy, thrombi were found in the femoral and iliac veins, as well as in the larger pulmonary arteries.
Thromboembolism: Testes

Thromboembolism: Bowel infarction
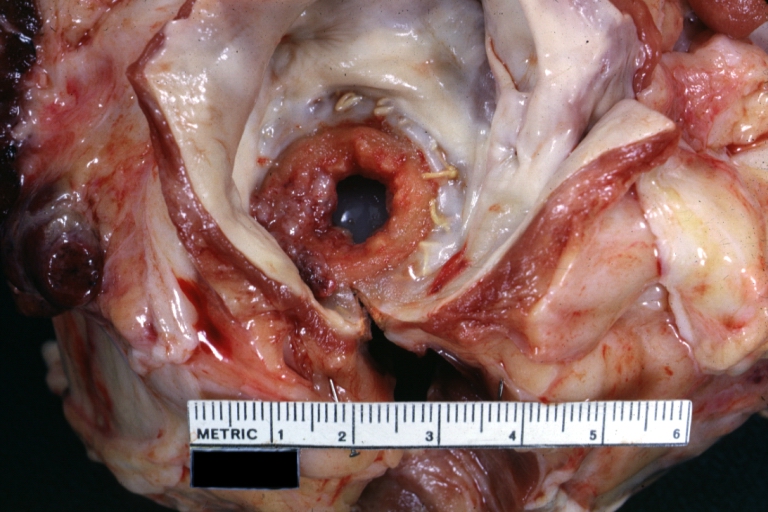
Coronary thrombosis
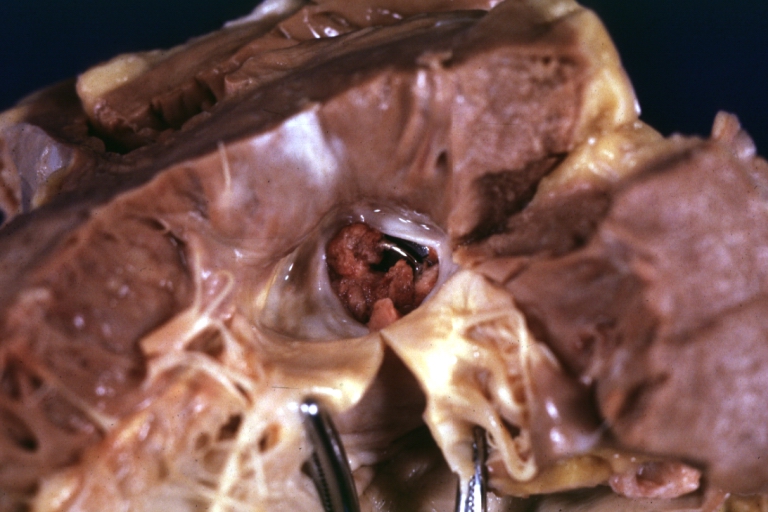
Artificial heart valve thrombosis
References
- ↑ Hellemans, Alexander (1988). The Timetables of Science. New York, New York: Simon and Schuster. p. 317. ISBN 0671621300. Unknown parameter
|coauthors=ignored (help) - ↑ Kuipers S, Cannegieter SC, Middeldorp S, Robyn L, Büller HR, et al. The Absolute Risk of Venous Thrombosis after Air Travel: A Cohort Study of 8,755 Employees of International Organisations PLoS Medicine Vol. 4, No. 9, e290 doi:10.1371/journal.PMID 0040290
- ↑ http://www.mounteverest.net/news.php?news=16349 Mount Everest experience
- ↑ Karlijn J. van Stralen, MSc; Frits R. Rosendaal, MD, PhD; Carine J. M. Doggen, PhD (January 14, 2008). "Minor Injuries as a Risk Factor for Venous Thrombosis". Arch Intern Med. 168 No. 1: 21–26. doi:10.1001/archinternmed.2007.5. PMID 18195191.
- ↑ Wells PS, Owen C, Doucette S, Fergusson D, Tran H (2006). "Does this patient have deep vein thrombosis?". JAMA. 295 (2): 199–207. doi:10.1001/jama.295.2.199. PMID 16403932.
- ↑ Scarvelis D, Wells P (2006). "Diagnosis and treatment of deep-vein thrombosis". CMAJ. 175 (9): 1087–92. PMID 17060659. Free Full Text.
- ↑ Neff MJ. ACEP releases clinical policy on evaluation and management of pulmonary embolism. American Family Physician. 2003; 68(4):759-?. Available at: http://www.aafp.org/afp/20030815/practice.html. Accessed on: December 8, 2006.
- ↑ 8.0 8.1 8.2 Wells PS, Anderson DR, Rodger M, Ginsberg JS, Kearon C, Gent M, Turpie AG, Bormanis J, Weitz J, Chamberlain M, Bowie D, Barnes D, Hirsh J. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000 Mar;83(3):416-20. PMID 10744147
- ↑ Wells PS, Anderson DR, Rodger M, Stiell I, Dreyer JF, Barnes D, Forgie M, Kovacs G, Ward J, Kovacs MJ. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and d-dimer. Ann Intern Med. 2001 Jul 17;135(2):98-107. PMID 11453709
- ↑ 10.0 10.1 10.2 Stein PD, Woodard PK, Weg JG, Wakefield TW, Tapson VF, Sostman HD, Sos TA, Quinn DA, Leeper KV, Hull RD, Hales CA, Gottschalk A, Goodman LR, Fowler SE, Buckley JD (2007). "Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II Investigators". Radiology. 242 (1): 15–21. doi:10.1148/radiol.2421060971. PMID 17185658.
- ↑ van Belle A, Büller HR, Huisman MV, Huisman PM, Kaasjager K, Kamphuisen PW, Kramer MH, Kruip MJ, Kwakkel-van Erp JM, Leebeek FW, Nijkeuter M, Prins MH, Sohne M, Tick LW; Christopher Study Investigators. Effectiveness of managing suspected pulmonary embolism using an algorithm combining clinical probability, D-dimer testing, and computed tomography. JAMA. 2006 Jan 11;295(2):172-9. PMID 16403929
- ↑ Bounameaux H, de Moerloose P, Perrier A, Reber G (1994). "Plasma measurement of D-dimer as diagnostic aid in suspected venous thromboembolism: an overview". Thromb. Haemost. 71 (1): 1–6. PMID 8165626.
- ↑ Othieno R, Abu Affan M, Okpo E (2007). "Home versus in-patient treatment for deep vein thrombosis". Cochrane database of systematic reviews (Online) (3): CD003076. doi:10.1002/14651858.CD003076.pub2. PMID 17636714.
- ↑ Trujillo-Santos J, Herrera S, Page MA; et al. (2006). "Predicting adverse outcome in outpatients with acute deep vein thrombosis. findings from the RIETE Registry". J. Vasc. Surg. 44 (4): 789–93. doi:10.1016/j.jvs.2006.06.032. PMID 16926081.
- ↑ 15.0 15.1 Snow V, Qaseem A, Barry P; et al. (2007). "Management of venous thromboembolism: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians". Ann. Intern. Med. 146 (3): 204–10. PMID 17261857.
- ↑ Hutten BA, Prins MH (2006). "Duration of treatment with vitamin K antagonists in symptomatic venous thromboembolism". Cochrane database of systematic reviews (Online) (1): CD001367. doi:10.1002/14651858.CD001367.pub2. PMID 16437432.
- ↑ Palareti G, Cosmi B, Legnani C; et al. (2006). "D-dimer testing to determine the duration of anticoagulation therapy". N. Engl. J. Med. 355 (17): 1780–9. doi:10.1056/NEJMoa054444. PMID 17065639.
- ↑ Watson L, Armon M. "Thrombolysis for acute deep vein thrombosis". Cochrane Database Syst Rev: CD002783. PMID 15495034.
- ↑ Decousus H, Leizorovicz A, Parent F, Page Y, Tardy B, Girard P, Laporte S, Faivre R, Charbonnier B, Barral F, Huet Y, Simonneau G (1998). "A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep-vein thrombosis. Prévention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group". N Engl J Med. 338 (7): 409–15. PMID 9459643.
- ↑ "Eight-year follow-up of patients with permanent vena cava filters in the prevention of pulmonary embolism: the PREPIC (Prevention du Risque d'Embolie Pulmonaire par Interruption Cave) randomized study". Circulation. 112 (3): 416–22. 2005. PMID 16009794.
- ↑ Young T, Aukes J, Hughes R, Tang H (2007). "Vena caval filters for the prevention of pulmonary embolism". Cochrane database of systematic reviews (Online) (3): CD006212. doi:10.1002/14651858.CD006212.pub2. PMID 17636834.
- ↑ Prandoni P, Lensing AW, Prins MH; et al. (2004). "Below-knee elastic compression stockings to prevent the post-thrombotic syndrome: a randomized, controlled trial". Ann. Intern. Med. 141 (4): 249–56. PMID 15313740.
- ↑ Kolbach D, Sandbrink M, Hamulyak K, Neumann H, Prins M. "Non-pharmaceutical measures for prevention of post-thrombotic syndrome". Cochrane Database Syst Rev: CD004174. doi:10.1002/14651858.CD004174.pub2. PMID 14974060.
- ↑ Kakkos S, Daskalopoulou S, Daskalopoulos M, Nicolaides A, Geroulakos G (2006). "Review on the value of graduated elastic compression stockings after deep vein thrombosis". Thromb Haemost. 96 (4): 441–5. PMID 17003920.
Additional Resources
- The MD TV: Comments on Hot Topics, State of the Art Presentations in Cardiovascular Medicine, Expert Reviews on Cardiovascular Research
- Clinical Trial Results: An up to dated resource of Cardiovascular Research
- Venous Disease Coalition
- The National Alliance for Thrombosis and Thrombophilia
See Also
- Atrial fibrillation
- Cerebrovascular accident
- Deep venous thrombosis
- Infective endocarditis
- Pulmonary embolism
- Thrombosis
- Embolism
External Links
- American Venous Forum
- American College of Phlebology
- Vascular Disease Foundation
- National Lymphedema Network
- MyLymphedema
Template:Skin and subcutaneous tissue symptoms and signs Template:Nervous and musculoskeletal system symptoms and signs Template:Urinary system symptoms and signs Template:Cognition, perception, emotional state and behaviour symptoms and signs Template:Speech and voice symptoms and signs Template:General symptoms and signs