Theophylline
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Theophylline is a anti-asthmatic that is FDA approved for the treatment of is indicated for the treatment of the symptoms and reversible airflow obstruction associated with chronic asthma and other chronic lung diseases, e.g., emphysema and chronic bronchitis.. Common adverse reactions include vomiting, diarrhea, abdominal pain, hematemesis, acid-base disturbances, rhabdomyolysis, supraventricular tachycardia, shock, nervousness, tremors, disorientation and seziures..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
General Considerations
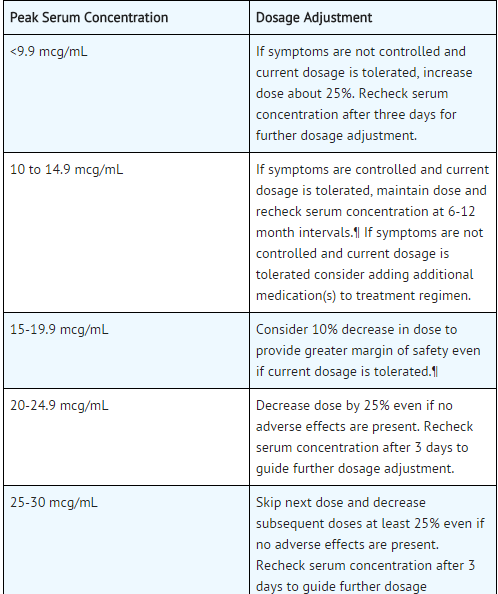
- The steady-state peak serum theophylline concentration is a function of the dose, the dosing interval, and the rate of theophylline absorption and clearance in the individual patient.
- Because of marked individual differences in the rate of theophylline clearance, the dose required to achieve a peak serum theophylline concentration in the 10-20 mcg/mL range varies fourfold among otherwise similar patients in the absence of factors known to alter theophylline clearance (e.g., 400-1600 mg/day in adults <60 years old and 10-36 mg/kg/day in children 1-9 years old).
- For a given population there is no single theophylline dose that will provide both safe and effective serum concentrations for all patients.
- Administration of the median theophylline dose required to achieve a therapeutic serum theophylline concentration in a given population may result in either sub-therapeutic or potentially toxic serum theophylline concentrations in individual patients.
- For example, at a dose of 900 mg/d in adults <60 years or 22 mg/kg/d in children 1-9 years, the steady state peak serum theophylline concentration will be <10 mcg/mL in about 30% of patients, 10-20 mcg/mL in about 50% and 20-30 mcg/mL in about 20% of patients.
- The dose of theophylline must be individualized on the basis of peak serum theophylline concentration measurements in order to achieve a dose that will provide maximum potential benefit with minimal risk to adverse effects.
- Transient caffeine-like adverse effects and excessive serum concentrations in slow metabolizers can be avoided in most patients by starting with a sufficiently low dose and slowly increasing the dose, if judged to be clinically indicated, in small increments (See Table V).
- Dose increases should only be made if the previous dosage is well tolerated and at intervals of no less than 3 days to allow serum theophylline concentrations to reach the new steady state. Dosage adjustment should be guided by serum theophylline concentration measurement.
- Health care providers should instruct patients and care givers to discontinue any dosage that causes adverse effects, to withhold the medication until these symptoms are gone and to then resume therapy at a lower, previously tolerated dosage.
- If the patient’s symptoms are well controlled, there are no apparent adverse effects, and no intervening factors that might alter dosage requirements , serum theophylline concentrations should be monitored at 6 month intervals for rapidly growing children and at yearly intervals for all others.
- In acutely ill patients, serum theophylline concentrations should be monitored at frequent intervals, e.g., every 24 hours.
- Theophylline distributes poorly into body fat, therefore, mg/kg dose should be calculated on the basis of ideal body weight.
- Table V contains theophylline dosing titration schema recommended for patients in various age groups and clinical circumstances. Table VI contains recommendations for theophylline dosage adjustment based upon serum theophylline concentrations.
- Application of these general dosing recommendations to individual patients must take into account the unique clinical characteristics of each patient.
- In general, these recommendations should serve as the upper limit for dosage adjustments in order to decrease the risk of potentially serious adverse events associated with unexpected large increases in serum theophylline concentration.
Infants <1 year old
Initial Dosage
- Premature Neonates
- < 24 days postnatal age; 1.0 mg/kg every 12 hr
- >24 days postnatal age; 1.5 mg/kg every 12 hr
- Full term infants and infants up to 52 weeks of age:
- Total daily dose (mg) = [(0.2 x age in weeks)+5.0] x (Kg body Wt).
- Up to age 26 weeks; divide dose into 3 equal amounts administered at 8 hour intervals.
- >26 weeks of age; divide dose into 4 equal amounts administered at 6 hour intervals.
- Final Dosage.
- Adjusted to maintain a peak steady state serum theophylline concentration of 5-10 mcg/ml in neonates and 10-15 mcg/mL in older infants (see Table VI). :* Since the time required to reach steady-state is a function of theophylline half-life, up to 5 days may be required to achieve steady state in a premature neonate while only 2-3 days may be required in a 6 month old infant without other risk factors for impaired clearance in the absence of a loading dose.
- If a serum theophylline concentration is obtained before steady state is achieved, the maintenance dose should not be increased, even if the serum theophylline concentration is <10 mcg/mL.
This image is provided by the National Library of Medicine.
Patients With Risk Factors For Impaired Clearance, The Elderly (>60 Years), And Those In Whom It Is Not Feasible To Monitor Serum Theophylline Concentrations
- In children 1-15 years of age, the final theophylline dose should not exceed 16 mg/kg/day up to a maximum of 400 mg/day in the presence of risk factors for reduced theophylline clearance (see WARNINGS) or if it is not feasible to monitor serum theophylline concentrations.
- In adolescents ≥16 years and adults, including the elderly, the final theophylline dose should not exceed 400 mg/day in the presence of risk factors for reduced theophylline clearance (see WARNINGS) or if it is not feasible to monitor serum theophylline concentrations.
Loading Dose for Acute Bronchodilatation
- An inhaled beta-2 selective agonist, alone or in combination with a systemically administered corticosteroid, is the most effective treatment for acute exacerbations of reversible airways obstruction.
- Theophylline is a relatively weak bronchodilator, is less effective than an inhaled beta-2 selective agonist and provides no added benefit in the treatment of acute bronchospasm.
- If an inhaled or parenteral beta agonist is not available, a loading dose of an oral immediate release theophylline can be used as a temporary measure. * A single 5 mg/kg dose of theophylline, in a patient who has not received any theophylline in the previous 24 hours, will produce an average peak serum theophylline concentration of 10 mcg/mL (range 5-15 mcg/mL).
- If dosing with theophylline is to be continued beyond the loading dose, the guidelines in Sections A.1.b., B.3, or C., above, should be utilized and serum theophylline concentration monitored at 24 hour intervals to adjust final dosage.
- Patients with more rapid metabolism, clinically identified by higher than average dose requirements, should receive a smaller dose more frequently to prevent breakthrough symptoms resulting from low trough concentrations before the next dose.
- A reliably absorbed slow-release formulation will decrease fluctuations and permit longer dosing intervals.
This image is provided by the National Library of Medicine. 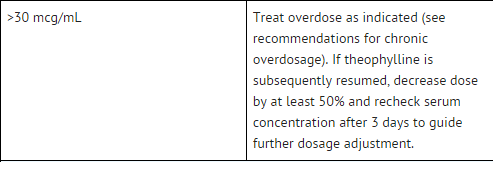
This image is provided by the National Library of Medicine.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Theophylline in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Theophylline in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Theophylline in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Theophylline in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Theophylline in pediatric patients.
Contraindications
- Condition1
Warnings
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Theophylline in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Theophylline in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Theophylline in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Theophylline during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Theophylline with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Theophylline with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Theophylline with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Theophylline with respect to specific gender populations.
Race
There is no FDA guidance on the use of Theophylline with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Theophylline in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Theophylline in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Theophylline in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Theophylline in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Theophylline in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Theophylline in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Theophylline in the drug label.
Pharmacology
There is limited information regarding Theophylline Pharmacology in the drug label.
Mechanism of Action
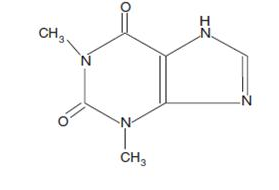
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Theophylline in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Theophylline in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Theophylline in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Theophylline in the drug label.
How Supplied
Storage
There is limited information regarding Theophylline Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Theophylline |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Theophylline |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Theophylline in the drug label.
Precautions with Alcohol
- Alcohol-Theophylline interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Theophylline
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Theophylline |Label Name=Theophylline11.png
}}
{{#subobject:
|Label Page=Theophylline |Label Name=Theophylline11.png
}}