Testosterone (transdermal): Difference between revisions
(Blanked the page) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
|authorTag={{SS}] | |||
|genericName=Testosterone | |||
|aOrAn=an | |||
|drugClass=Androgen | |||
|indicationType=treatment | |||
|indication=[[Primary hypogonadism]], [[Hypogonadotropic hypogonadism]] | |||
|adverseReactions=Acne, Scab of skin, Nasal, Gynecomastia, Oral irritation, Headache, Large prostate, Raised prostate specific antigen, Bronchitis, Discomfort, Nasal, Epistaxis, Nasal discharge, Nasopharyngitis, Sense of smell altered, Sinusitis, Upper respiratory infection | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |||
|fdaLIADAdult=<h4>Replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone</h4> | |||
* Dosing information | |||
:* Recommended starting dose: '''4 mg/day (not two 2 mg/day systems) applied nightly for 24 hours, 4 mg PO qd. To ensure proper dosing, approximately 2 weeks after starting therapy, the early morning serum testosterone concentration should be measured following system application the previous evening. Serum concentrations outside the range of 400 - 930 ng/dL require increasing the daily dose to '''6 mg''' (i.e., one '''4 mg/day''' and one '''2 mg/day''' system) or decreasing the daily dose to '''2 mg''',maintaining nightly application. | |||
:* Patients currently maintained on ANDRODERM 2.5 mg/day, 5 mg/day, and 7.5 mg/day may be switched to the 2 mg/day, 4 mg/day, and 6 mg/day dosage using the following schema: | |||
::* Patients using '''2.5 mg daily''' may be switched to '''2 mg/day''' systems at the next scheduled dose. | |||
::* Patients using '''5 mg daily''' may be switched to '''4 mg/day''' systems at the next scheduled dose. | |||
::* Patients using '''7.5 mg daily''' may be switched to '''6 mg''' (2 mg/day and 4 mg/day systems) at the next scheduled dose. | |||
:* To ensure proper dosing, approximately 2 weeks after switching therapy, the early morning serum testosterone concentration should be measured following system application the previous evening. | |||
:* The adhesive side of the ANDRODERM system should be applied to a clean, dry area of the skin on the back, abdomen, upper arms, or thighs. Avoid application over bony prominences or on a part of the body that may be subject to prolonged pressure during sleep or sitting (e.g., the deltoid region of the upper arm, the greater trochanter of the femur, and the ischial tuberosity). DO NOT APPLY TO THE SCROTUM. The sites of application should be rotated, with an interval of 7 days between applications to the same site. The area selected should not be oily, damaged, or irritated. | |||
:* The system should be applied immediately after opening the pouch and removing the protective release liner. The system should be pressed firmly in place, making sure there is good contact with the skin, especially around the edges. | |||
:* The patient should avoid swimming, showering, or washing the administration site for a minimum of 3 hours after application. | |||
:* Mild skin irritation may be ameliorated by treatment of the affected skin with over-the-counter topical hydrocortisone cream applied after system removal. Applying a small amount of 0.1% triamcinolone acetonide cream to the skin under the central drug reservoir of the ANDRODERM system has been shown to reduce the incidence and severity of skin irritation. | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Testosterone in adult patients. | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Testosterone in adult patients. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Testosterone in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Testosterone in pediatric patients. | |||
|contraindications=* ANDRODERM is contraindicated in men with [[carcinoma]] of the breast or known or suspected [[carcinoma]] of the prostate. | |||
* ANDRODERM is contraindicated in women who are, or who may become pregnant, or who are breastfeeding. ANDRODERM may cause fetal harm when administered to a pregnant woman. ANDRODERM may cause serious adverse reactions in nursing infants. If a pregnant woman is exposed to ANDRODERM, she should be apprised of the potential hazard to the fetus. | |||
|warnings=====Worsening of [[Benign Prostatic Hyperplasia]] and Potential Risk of [[Prostate Cancer]]==== | |||
Monitor patients with [[benign prostatic hyperplasia]] ([[BPH]]) for worsening of signs and symptoms of BPH. | |||
Patients treated with androgens may be at increased risk for [[prostate cancer]]. Evaluate patients for [[prostate cancer]] prior to initiating treatment. It is appropriate to re-evaluate patients 3 to 6 months after initiation of treatment, and then in accordance with [[prostate cancer]] screening practices. | |||
====[[Polycythemia]]==== | |||
Increases in hematocrit, reflective of increases in red blood cell mass, may require lowering or discontinuation of testosterone. Check hematocrit prior to initiating testosterone treatment. It is appropriate to re-evaluate the hematocrit 3 to 6 months after starting testosterone treatment, and then monitor annually. Discontinue testosterone therapy if the hematocrit becomes elevated. Testosterone therapy may be restarted when the hematocrit decreases to an acceptable level. An increase in red blood cell mass may increase the risk of thromboembolic events. | |||
====[[Venous Thromboembolism]]==== | |||
There have been postmarketing reports of venous thromboembolic events, including [[deep vein thrombosis]] ([[DVT]]) and [[pulmonary embolism]] ([[PE]]), in patients using testosterone products such as ANDRODERM. Evaluate patients who report symptoms of pain, [[edema]], warmth and erythema in the lower extremity for [[DVT]] and those who present with acute shortness of breath for [[PE]]. If a venous thromboembolic event is suspected, discontinue treatment with ANDRODERM and initiate appropriate workup and management. | |||
====Use in Women and Children==== | |||
Women and children should not use ANDRODERM. Use in women and children has not been studied with ANDRODERM. | |||
Due to lack of controlled studies in women and potential virilizing effects, ANDRODERM is not indicated for use in women and children. | |||
====Potential for Adverse Effects on [[Spermatogenesis]]==== | |||
At large doses of exogenous androgens, including ANDRODERM, [[spermatogenesis]] may be suppressed through feedback inhibition of [[pituitary follicle-stimulating hormone]] ([[FSH]]) that could lead to adverse effects on semen parameters including reduction of sperm count. | |||
====Hepatic Adverse Effects==== | |||
Prolonged use of high doses of orally active 17-alpha-alkyl androgens (methyltestosterone) has been associated with serious hepatic adverse effects ([[peliosis hepatis]], [[hepatic neoplasms]], [[cholestatic hepatitis]], and [[jaundice]]). [[Peliosis hepatis]] can be a life-threatening or fatal complication. Long-term therapy with intramuscular testosterone enanthate has produced multiple hepatic adenomas. ANDRODERM is not known to cause these adverse effects. | |||
====[[Edema]]==== | |||
Androgens, including ANDRODERM, may promote retention of sodium and water. [[Edema]], with or without [[congestive heart failure]], may be a serious complication in patients with pre-existing cardiac, renal, or hepatic disease. | |||
====[[Gynecomastia]]==== | |||
[[Gynecomastia]] may develop and persist in patients being treated with androgens, including ANDRODERM, for [[hypogonadism]]. | |||
====[[Sleep Apnea]]==== | |||
The treatment of hypogonadal men with testosterone may potentiate sleep apnea in some patients, especially those with risk factors such as obesity and chronic lung disease. | |||
====Lipids==== | |||
Changes in serum lipid profile may require dose adjustment or discontinuation of testosterone therapy. | |||
====[[Hypercalcemia]]==== | |||
Androgens, including ANDRODERM, should be used with caution in cancer patients at risk of [[hypercalcemia]] (and associated [[hypercalciuria]]). Regular monitoring of serum calcium concentrations is recommended in these patients. | |||
====Decreased Thyroxine-Binding Globulin==== | |||
Androgens, including ANDRODERM, may decrease concentrations of thyroxine-binding globulins, resulting in decreased total T4 serum concentration and increased resin uptake of T3 and T4. Free thyroid hormone concentration remains unchanged and there is no clinical evidence of thyroid dysfunction. | |||
====Magnetic Resonance Imaging (MRI)==== | |||
Skin burns have been reported at the application site in patients wearing an aluminized transdermal system during a magnetic resonance imaging scan (MRI). Because ANDRODERM contains aluminum, it is recommended to remove the system before undergoing an MRI. | |||
|clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
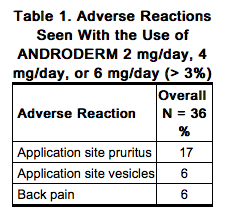
Table 1 shows the adverse reactions that were reported by > 3% of 36 hypogonadal men who were treated with ANDRODERM 2 mg/day, 4 mg/day, or 6 mg/day for 28 days. Of note, all hypogonadal men studied had been stable users of topical testosterone replacement products prior to the study and there was no washout period between therapies. Furthermore, there was only one subject titrated to 6 mg/day and he withdrew from the study prematurely. | |||
[[File:Testosterone_adverse_01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Other less common adverse reactions reported by < 3% of patients included: application site erythema, application site exfoliation, chills, diarrhea, fatigue, gastroesophageal reflux disease, hemarthrosis, hematuria, headache, polyuria, and prostatitis. The overall incidence of application site reactions of any kind was 28% (10 subjects with 13 adverse reactions). | |||
No serious adverse reactions to ANDRODERM 2 mg/day and 4 mg/day were reported during the clinical trial. | |||
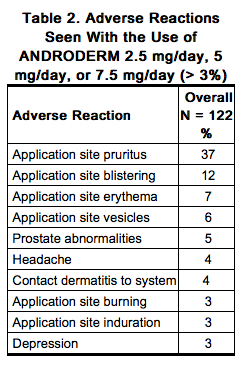
Table 2 shows the adverse reactions that were reported in > 3% of 122 patients in clinical studies with ANDRODERM dosage strengths of 2.5 mg/day, 5 mg/day, and 7.5 mg/day. The most common adverse reactions reported were application site reactions. Transient mild to moderate erythema was observed at the site of application in the majority of patients at some time during treatment. The overall incidence of application site reactions of any kind was 48% (59 subjects with 107 adverse reactions). | |||
[[File:Testosterone_adverse_02.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
The following reactions occurred in less than 3% of patients: rash, gastrointestinal bleeding, fatigue, body pain, pelvic pain, hypertension, peripheral vascular disease, increased appetite, accelerated growth, anxiety, confusion, decreased libido, paresthesia, thinking abnormalities, vertigo, acne, bullae at application site, mechanical irritation at application site, rash at application site, contamination of application site, prostate carcinoma, dysuria, hematuria, impotence, urinary incontinence, urinary tract infection, and testicular abnormalities. | |||
|postmarketing=The following adverse reactions have been identified during postapproval use of ANDRODERM. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
====Vascular Disorders:==== | |||
[[Venous thromboembolism]] | |||
|drugInteractions=====Insulin==== | |||
Changes in insulin sensitivity or glycemic control may occur in patients treated with androgens. In diabetic patients, the metabolic effects of androgens may decrease blood glucose and, therefore, insulin requirement. | |||
====Oral Anticoagulants==== | |||
Changes in anticoagulant activity may be seen with androgens. More frequent monitoring of INR and prothrombin time is recommended in patients taking anticoagulants, especially at the initiation and termination of androgen therapy. | |||
====[[Corticosteroids]]==== | |||
The concurrent use of testosterone with [[ACTH]] or [[corticosteroids]] may result in increased fluid retention and should be monitored, particularly in patients with cardiac, renal or hepatic disease. | |||
====[[Triamcinolone]]==== | |||
The topical administration of 0.1% triamcinolone cream to the skin under the central drug reservoir prior to the application of the ANDRODERM system did not significantly alter transdermal absorption of testosterone; however, the rate of complete adherence was lower. | |||
Pretreatment with triamcinolone ointment formulation significantly reduced testosterone absorption from the ANDRODERM system. | |||
|FDAPregCat=X | |||
|useInPregnancyFDA=Pregnancy Category X — ANDRODERM is contraindicated during pregnancy or in women who may become pregnant. Testosterone is teratogenic and may cause fetal harm. Exposure of a female fetus to androgens may result in varying degrees of virilization. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus. | |||
|useInNursing=Although it is not known how much testosterone transfers into human milk, ANDRODERM is contraindicated in nursing women because of the potential for serious adverse reactions in nursing infants. Testosterone and other androgens may adversely affect lactation. | |||
|useInPed=Safety and efficacy of ANDRODERM have not been established in males <18 years of age. Improper use may result in acceleration of bone age and premature closure of epiphyses. | |||
|useInGeri=There have not been sufficient numbers of geriatric patients involved in controlled clinical studies utilizing ANDRODERM to determine whether efficacy in those over 65 years of age differs from younger patients. Additionally, there are insufficient long-term safety data in geriatric patients utilizing ANDRODERM to assess a potential incremental risk of cardiovascular disease and prostate cancer. | |||
|useInRenalImpair=No studies were conducted in patients with renal impairment. | |||
|useInHepaticImpair=No studies were conducted in patients with hepatic impairment. | |||
|overdose=No cases of overdose with ANDRODERM have been reported in clinical trials. There is one report of acute overdosage by injection of testosterone enanthate: testosterone concentrations of up to 11,400 ng/dL were implicated in a cerebrovascular accident. Treatment of overdosage would consist of discontinuation of ANDRODERM together with appropriate symptomatic and supportive care. | |||
====Controlled Substance==== | |||
ANDRODERM contains testosterone, a Schedule III controlled substance under the Anabolic Steroids Control Act. | |||
====Abuse==== | |||
Anabolic steroids, such as testosterone, are abused. Abuse is often associated with adverse physical and psychological effects. | |||
====Dependence==== | |||
Although drug dependence is not documented in individuals using therapeutic doses of anabolic steroids for approved indications, dependence is observed in some individuals abusing high doses of anabolic steroids. In general, anabolic steroid dependence is characterized by any three of the following: | |||
* Taking more drug than intended | |||
* Continued drug use despite medical and social problems | |||
* Significant time spent in obtaining adequate amounts of drug | |||
* Desire for anabolic steroids when supplies of the drug are interrupted | |||
* Difficulty in discontinuing use of the drug despite desires and attempts to do so | |||
* Experience of withdrawal syndrome upon discontinuation of anabolic steroid use | |||
|drugBox={{Drugbox2| | |||
|IUPAC_name = (8R,9S,10R,13S,14S,17S)-17-hydroxy-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,1 | |||
6,17-dodecahydrocyclopenta[a]phenanthren-3-one | |||
| image=Testosterone structure.png | |||
| width=200px | |||
| smiles=C[C@]43CCC(=O)\C=C4\CC[C@@H]1[C@@H]3CC[C@]2(C)[C@@H](O)CC[C@@H]12 | |||
| CAS_number=58-22-0 | |||
| ATC_prefix=G03 | |||
| ATC_suffix=BA03 | |||
| ATC_supplemental= | |||
| PubChem=6013 | |||
| DrugBank= | |||
| C=19 | H=28 | O=2 | |||
| molecular_weight = 288.43 | |||
| bioavailability= low (due to extensive [[First pass effect|first pass metabolism]]) | |||
| metabolism = [[Liver]], [[Testis]] and [[Prostate]] | |||
| elimination_half-life= 2-4 hours | |||
| excretion = [[Urine]] (90%), feces (6%) | |||
| pregnancy_category = X ([[United States|USA]]), [[Teratogen]]ic effects | |||
| legal_status = Schedule III ([[Controlled Substances Act|USA]])<br />Schedule IV ([[Controlled Drugs and Substances Act|Canada]]) | |||
| routes_of_administration=Intramuscular injection, transdermal (cream, gel, or patch), sub-'Q' pellet | |||
| melting_point=155-156 | |||
| specific_rotation=+110,2° | |||
| sec_combustion=−11080 kJ/mol | |||
}} | |||
|mechAction=Endogenous androgens, including testosterone and dihydrotestosterone (DHT), are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of prostate, seminal vesicles, penis and scrotum; the development of male hair distribution, such as facial, pubic, chest and axillary hair; laryngeal enlargement; vocal cord thickening; and alterations in body musculature and fat distribution. Testosterone and DHT are necessary for the normal development of secondary sex characteristics. Male hypogonadism results from insufficient secretion of testosterone and is characterized by low serum testosterone concentrations. Signs/symptoms associated with male hypogonadism include erectile dysfunction and decreased sexual desire, fatigue and loss of energy, mood depression, regression of secondary sexual characteristics, and osteoporosis. | |||
Male hypogonadism has two main etiologies. Primary hypogonadism is caused by defects of the gonads, such as Klinefelter Syndrome or Leydig cell aplasia, whereas secondary hypogonadism is the failure of the hypothalamus (or pituitary) to produce sufficient gonadotropins (FSH, LH). | |||
|structure=ANDRODERM (testosterone transdermal system) is designed to deliver testosterone continuously for 24 hours following application to intact, non-scrotal skin (e.g., back, abdomen, thighs, upper arms). | |||
Two strengths of ANDRODERM are available that deliver approximately 2 mg or 4 mg of testosterone per day. | |||
ANDRODERM has a central drug delivery reservoir surrounded by a peripheral adhesive area. The ANDRODERM 2 mg/day system has a total contact surface area of 32 cm2 with a 6.0 cm2 central drug delivery reservoir containing 9.7 mg testosterone USP, dissolved in an alcohol-based gel. The ANDRODERM 4 mg/day system has a total contact surface area of 39 cm2 with a 12.0 cm2 central drug delivery reservoir containing 19.5 mg testosterone USP, dissolved in an alcohol-based gel. Testosterone USP is a white, or creamy white crystalline powder or crystals chemically described as 17ß-hydroxyandrost-4-en-3-one. | |||
[[File:Testosterone_structure_01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
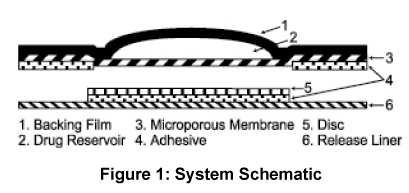
The ANDRODERM systems have six components as shown in Figure 1. Proceeding from the top toward the surface attached to the skin, the system is composed of (1) metallized polyester/Surlyn® (ethylene-methacrylic acid copolymer)/ethylene vinyl acetate backing film with alcohol resistant ink, (2) a drug reservoir of testosterone USP, alcohol USP, glycerin USP, glycerol monooleate, methyl laurate, sodium hydroxide NF, to adjust pH, and purified water USP, gelled with carbomer copolymer Type B NF, (3) a permeable polyethylene microporous membrane, and (4) a peripheral layer of acrylic adhesive surrounding the central, active drug delivery area of the system. Prior to opening of the system and application to the skin, the central delivery surface of the system is sealed with a peelable laminate disc (5) composed of a five-layer laminate containing polyester/polyesterurethane adhesive/aluminum foil/polyester-urethane adhesive/polyethylene. The disc is attached to and removed with the release liner (6), a silicone-coated polyester film, which is removed before the system can be used. | |||
[[File:Testosterone_structure_02.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
The active ingredient in the system is testosterone. The remaining components of the system are pharmacologically inactive. | |||
|PD=No specific pharmacodynamic studies were conducted using ANDRODERM. | |||
|PK=====Absorption==== | |||
ANDRODERM delivers physiologic amounts of testosterone, producing circulating testosterone concentrations that approximate the normal concentration range (300 - 1030 ng/dL) seen in healthy men. ANDRODERM provides a continuous daily dose of testosterone in a self-contained transdermal system. Following ANDRODERM application, testosterone is continuously absorbed during the 24-hour dosing period with a median (range) Tmax of 8 (4-12) hours. | |||
====Distribution==== | |||
Circulating testosterone is primarily bound in the serum to sex hormone-binding globulin (SHBG) and albumin. Approximately 40% of testosterone in plasma is bound to SHBG, 2% remains unbound (free) and the rest is bound to albumin and other proteins. | |||
====Metabolism==== | |||
Testosterone is metabolized to various 17-keto steroids through two different pathways. The major active metabolites of testosterone are estradiol and dihydrotestosterone (DHT). | |||
During steady-state pharmacokinetic studies in hypogonadal men treated with ANDRODERM, the average DHT:T and E2:T ratios were approximately 1:10 and 1:200, respectively. | |||
====Excretion==== | |||
There is considerable variation in the half-life of testosterone as reported in the literature, ranging from 10 to 100 minutes. About 90% of a dose of testosterone given intramuscularly is excreted in the urine as glucuronic and sulfuric acid conjugates of testosterone and its metabolites; about 6% of a dose is excreted in the feces, mostly in the unconjugated form. Inactivation of testosterone occurs primarily in the liver. | |||
Upon removal of the ANDRODERM systems, serum testosterone concentrations decrease with an apparent half-life of approximately 70 minutes. Hypogonadal concentrations are reached within 24 hours following system removal. There is no accumulation of testosterone during continuous treatment. | |||
====Effect of Showering==== | |||
In a two-way crossover study, the effects of showering on the pharmacokinetics of total testosterone following a single application of ANDRODERM 4 mg/day were assessed in 16 hypogonadal males. Showering 3 hours after application of ANDRODERM increased Cavg by 0.5% and decreased Cmax by 0.4% respectively, as compared to not showering. The systemic exposure to ANDRODERM was similar following applications with or without showering 3 hours after application. | |||
|nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility==== | |||
Testosterone has been tested by subcutaneous injection and implantation in mice and rats. In mice, the implant induced cervical-uterine tumors, which metastasized in some cases. There is suggestive evidence that injection of testosterone into some strains of female mice increases their susceptibility to hepatoma. Testosterone is also known to increase the number of tumors and decrease the degree of differentiation of chemically induced carcinomas of the liver in rats. Testosterone was negative in the in vitro Ames and in the in vivo mouse micronucleus assays. The administration of exogenous testosterone has been reported to suppress spermatogenesis in the rat, dog and non-human primates, which was reversible on cessation of the treatment. | |||
|clinicalStudies=ANDRODERM 2 mg/day and 4 mg/day were studied in a trial designed to evaluate the use and titration of 2 mg/day and 4 mg/day systems in a clinic setting of 40 men with hypogonadism. Thirty-eight of the 40 subjects (95%) who were enrolled into the study were white and 2 subjects were African American. Ten (25%) subjects were Hispanic and 30 (75%) were Non-Hispanic. Men were between 34 and 76 years of age (mean: 55 years). Patients had previously been on stable therapy of ANDRODERM 5 mg; Androgel® 2.5 g, 5 g, 7.5 g or 10 g; or Testim® 2.5 g or 5 g daily before switching to ANDRODERM 4 mg/day. | |||
Patients applied an ANDRODERM 4 mg/day system around 10 p.m. once daily for 14 days, and then were titrated up to 6 mg/day or down to 2 mg/day according to a morning serum testosterone concentration obtained at 6 a.m. on Day 8. Out of 36 patients who entered the study, 31 (86%) patients remained on the 4 mg/day dose, 4 (11%) were titrated downward to 2 mg/day, and 1 (3%) was titrated upward to 6 mg/day based on the Day 8 testosterone concentrations. The one patient that was titrated to 6 mg/day discontinued from the study for a non-safety related reason. Of the patients who were receiving ANDRODERM 5 mg/day prior to study entry (n = 11), 10 remained at 4 mg/day after titration, and 1 was titrated down to the 2 mg/day dose. | |||
After a total of 28 days of therapy, 34 of the 35 subjects (97%) had serum testosterone Cavg within the normal range during the dosing period, with the lower bound of the 95% confidence interval for this estimate being 85% (Table 3). One subject who received ANDRODERM 4 mg/day treatment had serum testosterone Cavg below 300 ng/dL and none had Cavg concentrations above 1030 ng/dL. The mean (SD) serum testosterone Cmax following treatment with the 2 mg/day (N = 4) and 4 mg/day (N = 31) systems was 648 (145) ng/dL and 696 (158) ng/dL, respectively. Table 3 summarizes testosterone Cavg categories by treatment. | |||
[[File:Testosterone_clinical studies_01.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
Figure 2 summarizes the pharmacokinetic profiles of total testosterone in 35 patients completing 28 days of ANDRODERM treatment applied as a starting dose of 4 mg/day for the initial 14 days followed by a possible dose titration. | |||
[[File:Testosterone_clinical studies_02.png|thumb|none|400px|This image is provided by the National Library of Medicine.]] | |||
In separate clinical studies using the ANDRODERM 2.5 mg/day system, 1% used 2.5 mg daily, 93% of patients used 5 mg daily, and 6% used 7.5 mg daily. The hormonal effects of ANDRODERM 2.5 mg/day system as a treatment for male hypogonadism was demonstrated in four open-label trials that included 94 hypogonadal men, ages 15 to 65 years. In these trials, ANDRODERM produced average morning serum testosterone concentrations within the normal reference range in 92% of patients. | |||
|howSupplied=ANDRODERM (testosterone transdermal system) 2 mg/day. | |||
Each system contains 9.7 mg testosterone USP for delivery of 2 mg of testosterone per day [see Description (11)]. | |||
Cartons of 60 systems NDC 52544-076-60 | |||
ANDRODERM (testosterone transdermal system) 4 mg/day. | |||
Each system contains 19.5 mg testosterone USP for delivery of 4 mg of testosterone per day [see Description (11)]. | |||
Cartons of 30 systems NDC 52544-077-30 | |||
|storage=Store at 20-25°C (68-77°F). [See USP controlled room temperature.] Apply to skin immediately upon removal from the protective pouch. Do not store outside the pouch provided. Damaged systems should not be used. The drug reservoir may be burst by excessive pressure or heat. Discard systems in household trash in a manner that prevents accidental application or ingestion by children, pets or others. | |||
|alcohol=Alcohol-Testosterone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
}} | |||
Revision as of 18:15, 28 August 2014
{{DrugProjectFormSinglePage |authorTag={{SS}] |genericName=Testosterone |aOrAn=an |drugClass=Androgen |indicationType=treatment |indication=Primary hypogonadism, Hypogonadotropic hypogonadism |adverseReactions=Acne, Scab of skin, Nasal, Gynecomastia, Oral irritation, Headache, Large prostate, Raised prostate specific antigen, Bronchitis, Discomfort, Nasal, Epistaxis, Nasal discharge, Nasopharyngitis, Sense of smell altered, Sinusitis, Upper respiratory infection |blackBoxWarningTitle=TITLE |blackBoxWarningBody=Condition Name: (Content)
|fdaLIADAdult=
Replacement therapy in adult males for conditions associated with a deficiency or absence of endogenous testosterone
- Dosing information
- Recommended starting dose: 4 mg/day (not two 2 mg/day systems) applied nightly for 24 hours, 4 mg PO qd. To ensure proper dosing, approximately 2 weeks after starting therapy, the early morning serum testosterone concentration should be measured following system application the previous evening. Serum concentrations outside the range of 400 - 930 ng/dL require increasing the daily dose to 6 mg (i.e., one 4 mg/day and one 2 mg/day system) or decreasing the daily dose to 2 mg,maintaining nightly application.
- Patients currently maintained on ANDRODERM 2.5 mg/day, 5 mg/day, and 7.5 mg/day may be switched to the 2 mg/day, 4 mg/day, and 6 mg/day dosage using the following schema:
- Patients using 2.5 mg daily may be switched to 2 mg/day systems at the next scheduled dose.
- Patients using 5 mg daily may be switched to 4 mg/day systems at the next scheduled dose.
- Patients using 7.5 mg daily may be switched to 6 mg (2 mg/day and 4 mg/day systems) at the next scheduled dose.
- To ensure proper dosing, approximately 2 weeks after switching therapy, the early morning serum testosterone concentration should be measured following system application the previous evening.
- The adhesive side of the ANDRODERM system should be applied to a clean, dry area of the skin on the back, abdomen, upper arms, or thighs. Avoid application over bony prominences or on a part of the body that may be subject to prolonged pressure during sleep or sitting (e.g., the deltoid region of the upper arm, the greater trochanter of the femur, and the ischial tuberosity). DO NOT APPLY TO THE SCROTUM. The sites of application should be rotated, with an interval of 7 days between applications to the same site. The area selected should not be oily, damaged, or irritated.
- The system should be applied immediately after opening the pouch and removing the protective release liner. The system should be pressed firmly in place, making sure there is good contact with the skin, especially around the edges.
- The patient should avoid swimming, showering, or washing the administration site for a minimum of 3 hours after application.
- Mild skin irritation may be ameliorated by treatment of the affected skin with over-the-counter topical hydrocortisone cream applied after system removal. Applying a small amount of 0.1% triamcinolone acetonide cream to the skin under the central drug reservoir of the ANDRODERM system has been shown to reduce the incidence and severity of skin irritation.
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Testosterone in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Testosterone in adult patients. |offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Testosterone in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Testosterone in pediatric patients. |contraindications=* ANDRODERM is contraindicated in men with carcinoma of the breast or known or suspected carcinoma of the prostate.
- ANDRODERM is contraindicated in women who are, or who may become pregnant, or who are breastfeeding. ANDRODERM may cause fetal harm when administered to a pregnant woman. ANDRODERM may cause serious adverse reactions in nursing infants. If a pregnant woman is exposed to ANDRODERM, she should be apprised of the potential hazard to the fetus.
|warnings=====Worsening of Benign Prostatic Hyperplasia and Potential Risk of Prostate Cancer====
Monitor patients with benign prostatic hyperplasia (BPH) for worsening of signs and symptoms of BPH. Patients treated with androgens may be at increased risk for prostate cancer. Evaluate patients for prostate cancer prior to initiating treatment. It is appropriate to re-evaluate patients 3 to 6 months after initiation of treatment, and then in accordance with prostate cancer screening practices.
Polycythemia
Increases in hematocrit, reflective of increases in red blood cell mass, may require lowering or discontinuation of testosterone. Check hematocrit prior to initiating testosterone treatment. It is appropriate to re-evaluate the hematocrit 3 to 6 months after starting testosterone treatment, and then monitor annually. Discontinue testosterone therapy if the hematocrit becomes elevated. Testosterone therapy may be restarted when the hematocrit decreases to an acceptable level. An increase in red blood cell mass may increase the risk of thromboembolic events.
Venous Thromboembolism
There have been postmarketing reports of venous thromboembolic events, including deep vein thrombosis (DVT) and pulmonary embolism (PE), in patients using testosterone products such as ANDRODERM. Evaluate patients who report symptoms of pain, edema, warmth and erythema in the lower extremity for DVT and those who present with acute shortness of breath for PE. If a venous thromboembolic event is suspected, discontinue treatment with ANDRODERM and initiate appropriate workup and management.
Use in Women and Children
Women and children should not use ANDRODERM. Use in women and children has not been studied with ANDRODERM. Due to lack of controlled studies in women and potential virilizing effects, ANDRODERM is not indicated for use in women and children.
Potential for Adverse Effects on Spermatogenesis
At large doses of exogenous androgens, including ANDRODERM, spermatogenesis may be suppressed through feedback inhibition of pituitary follicle-stimulating hormone (FSH) that could lead to adverse effects on semen parameters including reduction of sperm count.
Hepatic Adverse Effects
Prolonged use of high doses of orally active 17-alpha-alkyl androgens (methyltestosterone) has been associated with serious hepatic adverse effects (peliosis hepatis, hepatic neoplasms, cholestatic hepatitis, and jaundice). Peliosis hepatis can be a life-threatening or fatal complication. Long-term therapy with intramuscular testosterone enanthate has produced multiple hepatic adenomas. ANDRODERM is not known to cause these adverse effects.
Edema
Androgens, including ANDRODERM, may promote retention of sodium and water. Edema, with or without congestive heart failure, may be a serious complication in patients with pre-existing cardiac, renal, or hepatic disease.
Gynecomastia
Gynecomastia may develop and persist in patients being treated with androgens, including ANDRODERM, for hypogonadism.
Sleep Apnea
The treatment of hypogonadal men with testosterone may potentiate sleep apnea in some patients, especially those with risk factors such as obesity and chronic lung disease.
Lipids
Changes in serum lipid profile may require dose adjustment or discontinuation of testosterone therapy.
Hypercalcemia
Androgens, including ANDRODERM, should be used with caution in cancer patients at risk of hypercalcemia (and associated hypercalciuria). Regular monitoring of serum calcium concentrations is recommended in these patients.
Decreased Thyroxine-Binding Globulin
Androgens, including ANDRODERM, may decrease concentrations of thyroxine-binding globulins, resulting in decreased total T4 serum concentration and increased resin uptake of T3 and T4. Free thyroid hormone concentration remains unchanged and there is no clinical evidence of thyroid dysfunction.
Magnetic Resonance Imaging (MRI)
Skin burns have been reported at the application site in patients wearing an aluminized transdermal system during a magnetic resonance imaging scan (MRI). Because ANDRODERM contains aluminum, it is recommended to remove the system before undergoing an MRI. |clinicalTrials=Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice. Table 1 shows the adverse reactions that were reported by > 3% of 36 hypogonadal men who were treated with ANDRODERM 2 mg/day, 4 mg/day, or 6 mg/day for 28 days. Of note, all hypogonadal men studied had been stable users of topical testosterone replacement products prior to the study and there was no washout period between therapies. Furthermore, there was only one subject titrated to 6 mg/day and he withdrew from the study prematurely.
Other less common adverse reactions reported by < 3% of patients included: application site erythema, application site exfoliation, chills, diarrhea, fatigue, gastroesophageal reflux disease, hemarthrosis, hematuria, headache, polyuria, and prostatitis. The overall incidence of application site reactions of any kind was 28% (10 subjects with 13 adverse reactions). No serious adverse reactions to ANDRODERM 2 mg/day and 4 mg/day were reported during the clinical trial. Table 2 shows the adverse reactions that were reported in > 3% of 122 patients in clinical studies with ANDRODERM dosage strengths of 2.5 mg/day, 5 mg/day, and 7.5 mg/day. The most common adverse reactions reported were application site reactions. Transient mild to moderate erythema was observed at the site of application in the majority of patients at some time during treatment. The overall incidence of application site reactions of any kind was 48% (59 subjects with 107 adverse reactions).
The following reactions occurred in less than 3% of patients: rash, gastrointestinal bleeding, fatigue, body pain, pelvic pain, hypertension, peripheral vascular disease, increased appetite, accelerated growth, anxiety, confusion, decreased libido, paresthesia, thinking abnormalities, vertigo, acne, bullae at application site, mechanical irritation at application site, rash at application site, contamination of application site, prostate carcinoma, dysuria, hematuria, impotence, urinary incontinence, urinary tract infection, and testicular abnormalities.
|postmarketing=The following adverse reactions have been identified during postapproval use of ANDRODERM. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Vascular Disorders:
Venous thromboembolism |drugInteractions=====Insulin====
Changes in insulin sensitivity or glycemic control may occur in patients treated with androgens. In diabetic patients, the metabolic effects of androgens may decrease blood glucose and, therefore, insulin requirement.
Oral Anticoagulants
Changes in anticoagulant activity may be seen with androgens. More frequent monitoring of INR and prothrombin time is recommended in patients taking anticoagulants, especially at the initiation and termination of androgen therapy.
Corticosteroids
The concurrent use of testosterone with ACTH or corticosteroids may result in increased fluid retention and should be monitored, particularly in patients with cardiac, renal or hepatic disease.
Triamcinolone
The topical administration of 0.1% triamcinolone cream to the skin under the central drug reservoir prior to the application of the ANDRODERM system did not significantly alter transdermal absorption of testosterone; however, the rate of complete adherence was lower. Pretreatment with triamcinolone ointment formulation significantly reduced testosterone absorption from the ANDRODERM system. |FDAPregCat=X |useInPregnancyFDA=Pregnancy Category X — ANDRODERM is contraindicated during pregnancy or in women who may become pregnant. Testosterone is teratogenic and may cause fetal harm. Exposure of a female fetus to androgens may result in varying degrees of virilization. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus. |useInNursing=Although it is not known how much testosterone transfers into human milk, ANDRODERM is contraindicated in nursing women because of the potential for serious adverse reactions in nursing infants. Testosterone and other androgens may adversely affect lactation. |useInPed=Safety and efficacy of ANDRODERM have not been established in males <18 years of age. Improper use may result in acceleration of bone age and premature closure of epiphyses.
|useInGeri=There have not been sufficient numbers of geriatric patients involved in controlled clinical studies utilizing ANDRODERM to determine whether efficacy in those over 65 years of age differs from younger patients. Additionally, there are insufficient long-term safety data in geriatric patients utilizing ANDRODERM to assess a potential incremental risk of cardiovascular disease and prostate cancer. |useInRenalImpair=No studies were conducted in patients with renal impairment.
|useInHepaticImpair=No studies were conducted in patients with hepatic impairment. |overdose=No cases of overdose with ANDRODERM have been reported in clinical trials. There is one report of acute overdosage by injection of testosterone enanthate: testosterone concentrations of up to 11,400 ng/dL were implicated in a cerebrovascular accident. Treatment of overdosage would consist of discontinuation of ANDRODERM together with appropriate symptomatic and supportive care.
Controlled Substance
ANDRODERM contains testosterone, a Schedule III controlled substance under the Anabolic Steroids Control Act.
Abuse
Anabolic steroids, such as testosterone, are abused. Abuse is often associated with adverse physical and psychological effects.
Dependence
Although drug dependence is not documented in individuals using therapeutic doses of anabolic steroids for approved indications, dependence is observed in some individuals abusing high doses of anabolic steroids. In general, anabolic steroid dependence is characterized by any three of the following:
- Taking more drug than intended
- Continued drug use despite medical and social problems
- Significant time spent in obtaining adequate amounts of drug
- Desire for anabolic steroids when supplies of the drug are interrupted
- Difficulty in discontinuing use of the drug despite desires and attempts to do so
- Experience of withdrawal syndrome upon discontinuation of anabolic steroid use
|drugBox=
|mechAction=Endogenous androgens, including testosterone and dihydrotestosterone (DHT), are responsible for the normal growth and development of the male sex organs and for maintenance of secondary sex characteristics. These effects include the growth and maturation of prostate, seminal vesicles, penis and scrotum; the development of male hair distribution, such as facial, pubic, chest and axillary hair; laryngeal enlargement; vocal cord thickening; and alterations in body musculature and fat distribution. Testosterone and DHT are necessary for the normal development of secondary sex characteristics. Male hypogonadism results from insufficient secretion of testosterone and is characterized by low serum testosterone concentrations. Signs/symptoms associated with male hypogonadism include erectile dysfunction and decreased sexual desire, fatigue and loss of energy, mood depression, regression of secondary sexual characteristics, and osteoporosis. Male hypogonadism has two main etiologies. Primary hypogonadism is caused by defects of the gonads, such as Klinefelter Syndrome or Leydig cell aplasia, whereas secondary hypogonadism is the failure of the hypothalamus (or pituitary) to produce sufficient gonadotropins (FSH, LH). |structure=ANDRODERM (testosterone transdermal system) is designed to deliver testosterone continuously for 24 hours following application to intact, non-scrotal skin (e.g., back, abdomen, thighs, upper arms). Two strengths of ANDRODERM are available that deliver approximately 2 mg or 4 mg of testosterone per day. ANDRODERM has a central drug delivery reservoir surrounded by a peripheral adhesive area. The ANDRODERM 2 mg/day system has a total contact surface area of 32 cm2 with a 6.0 cm2 central drug delivery reservoir containing 9.7 mg testosterone USP, dissolved in an alcohol-based gel. The ANDRODERM 4 mg/day system has a total contact surface area of 39 cm2 with a 12.0 cm2 central drug delivery reservoir containing 19.5 mg testosterone USP, dissolved in an alcohol-based gel. Testosterone USP is a white, or creamy white crystalline powder or crystals chemically described as 17ß-hydroxyandrost-4-en-3-one.

The ANDRODERM systems have six components as shown in Figure 1. Proceeding from the top toward the surface attached to the skin, the system is composed of (1) metallized polyester/Surlyn® (ethylene-methacrylic acid copolymer)/ethylene vinyl acetate backing film with alcohol resistant ink, (2) a drug reservoir of testosterone USP, alcohol USP, glycerin USP, glycerol monooleate, methyl laurate, sodium hydroxide NF, to adjust pH, and purified water USP, gelled with carbomer copolymer Type B NF, (3) a permeable polyethylene microporous membrane, and (4) a peripheral layer of acrylic adhesive surrounding the central, active drug delivery area of the system. Prior to opening of the system and application to the skin, the central delivery surface of the system is sealed with a peelable laminate disc (5) composed of a five-layer laminate containing polyester/polyesterurethane adhesive/aluminum foil/polyester-urethane adhesive/polyethylene. The disc is attached to and removed with the release liner (6), a silicone-coated polyester film, which is removed before the system can be used.
The active ingredient in the system is testosterone. The remaining components of the system are pharmacologically inactive.
|PD=No specific pharmacodynamic studies were conducted using ANDRODERM. |PK=====Absorption====
ANDRODERM delivers physiologic amounts of testosterone, producing circulating testosterone concentrations that approximate the normal concentration range (300 - 1030 ng/dL) seen in healthy men. ANDRODERM provides a continuous daily dose of testosterone in a self-contained transdermal system. Following ANDRODERM application, testosterone is continuously absorbed during the 24-hour dosing period with a median (range) Tmax of 8 (4-12) hours.
Distribution
Circulating testosterone is primarily bound in the serum to sex hormone-binding globulin (SHBG) and albumin. Approximately 40% of testosterone in plasma is bound to SHBG, 2% remains unbound (free) and the rest is bound to albumin and other proteins.
Metabolism
Testosterone is metabolized to various 17-keto steroids through two different pathways. The major active metabolites of testosterone are estradiol and dihydrotestosterone (DHT). During steady-state pharmacokinetic studies in hypogonadal men treated with ANDRODERM, the average DHT:T and E2:T ratios were approximately 1:10 and 1:200, respectively.
Excretion
There is considerable variation in the half-life of testosterone as reported in the literature, ranging from 10 to 100 minutes. About 90% of a dose of testosterone given intramuscularly is excreted in the urine as glucuronic and sulfuric acid conjugates of testosterone and its metabolites; about 6% of a dose is excreted in the feces, mostly in the unconjugated form. Inactivation of testosterone occurs primarily in the liver. Upon removal of the ANDRODERM systems, serum testosterone concentrations decrease with an apparent half-life of approximately 70 minutes. Hypogonadal concentrations are reached within 24 hours following system removal. There is no accumulation of testosterone during continuous treatment.
Effect of Showering
In a two-way crossover study, the effects of showering on the pharmacokinetics of total testosterone following a single application of ANDRODERM 4 mg/day were assessed in 16 hypogonadal males. Showering 3 hours after application of ANDRODERM increased Cavg by 0.5% and decreased Cmax by 0.4% respectively, as compared to not showering. The systemic exposure to ANDRODERM was similar following applications with or without showering 3 hours after application. |nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility====
Testosterone has been tested by subcutaneous injection and implantation in mice and rats. In mice, the implant induced cervical-uterine tumors, which metastasized in some cases. There is suggestive evidence that injection of testosterone into some strains of female mice increases their susceptibility to hepatoma. Testosterone is also known to increase the number of tumors and decrease the degree of differentiation of chemically induced carcinomas of the liver in rats. Testosterone was negative in the in vitro Ames and in the in vivo mouse micronucleus assays. The administration of exogenous testosterone has been reported to suppress spermatogenesis in the rat, dog and non-human primates, which was reversible on cessation of the treatment. |clinicalStudies=ANDRODERM 2 mg/day and 4 mg/day were studied in a trial designed to evaluate the use and titration of 2 mg/day and 4 mg/day systems in a clinic setting of 40 men with hypogonadism. Thirty-eight of the 40 subjects (95%) who were enrolled into the study were white and 2 subjects were African American. Ten (25%) subjects were Hispanic and 30 (75%) were Non-Hispanic. Men were between 34 and 76 years of age (mean: 55 years). Patients had previously been on stable therapy of ANDRODERM 5 mg; Androgel® 2.5 g, 5 g, 7.5 g or 10 g; or Testim® 2.5 g or 5 g daily before switching to ANDRODERM 4 mg/day. Patients applied an ANDRODERM 4 mg/day system around 10 p.m. once daily for 14 days, and then were titrated up to 6 mg/day or down to 2 mg/day according to a morning serum testosterone concentration obtained at 6 a.m. on Day 8. Out of 36 patients who entered the study, 31 (86%) patients remained on the 4 mg/day dose, 4 (11%) were titrated downward to 2 mg/day, and 1 (3%) was titrated upward to 6 mg/day based on the Day 8 testosterone concentrations. The one patient that was titrated to 6 mg/day discontinued from the study for a non-safety related reason. Of the patients who were receiving ANDRODERM 5 mg/day prior to study entry (n = 11), 10 remained at 4 mg/day after titration, and 1 was titrated down to the 2 mg/day dose. After a total of 28 days of therapy, 34 of the 35 subjects (97%) had serum testosterone Cavg within the normal range during the dosing period, with the lower bound of the 95% confidence interval for this estimate being 85% (Table 3). One subject who received ANDRODERM 4 mg/day treatment had serum testosterone Cavg below 300 ng/dL and none had Cavg concentrations above 1030 ng/dL. The mean (SD) serum testosterone Cmax following treatment with the 2 mg/day (N = 4) and 4 mg/day (N = 31) systems was 648 (145) ng/dL and 696 (158) ng/dL, respectively. Table 3 summarizes testosterone Cavg categories by treatment.
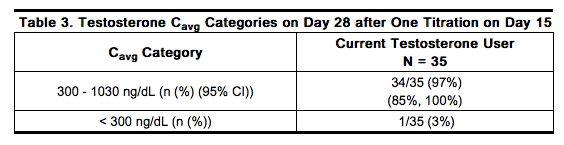
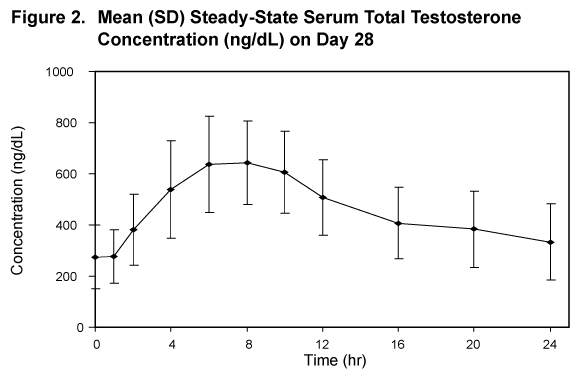
Figure 2 summarizes the pharmacokinetic profiles of total testosterone in 35 patients completing 28 days of ANDRODERM treatment applied as a starting dose of 4 mg/day for the initial 14 days followed by a possible dose titration.
In separate clinical studies using the ANDRODERM 2.5 mg/day system, 1% used 2.5 mg daily, 93% of patients used 5 mg daily, and 6% used 7.5 mg daily. The hormonal effects of ANDRODERM 2.5 mg/day system as a treatment for male hypogonadism was demonstrated in four open-label trials that included 94 hypogonadal men, ages 15 to 65 years. In these trials, ANDRODERM produced average morning serum testosterone concentrations within the normal reference range in 92% of patients.
|howSupplied=ANDRODERM (testosterone transdermal system) 2 mg/day. Each system contains 9.7 mg testosterone USP for delivery of 2 mg of testosterone per day [see Description (11)]. Cartons of 60 systems NDC 52544-076-60 ANDRODERM (testosterone transdermal system) 4 mg/day. Each system contains 19.5 mg testosterone USP for delivery of 4 mg of testosterone per day [see Description (11)]. Cartons of 30 systems NDC 52544-077-30 |storage=Store at 20-25°C (68-77°F). [See USP controlled room temperature.] Apply to skin immediately upon removal from the protective pouch. Do not store outside the pouch provided. Damaged systems should not be used. The drug reservoir may be burst by excessive pressure or heat. Discard systems in household trash in a manner that prevents accidental application or ingestion by children, pets or others. |alcohol=Alcohol-Testosterone interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. }}