Terguride: Difference between revisions
(Created page with "{{Drugbox | verifiedrevid = 437134889 | IUPAC_name = ''N'',''N''-diethyl-''N'''-[(8α)-6-methylergolin-8-yl]urea | image = Terguride.png <!--Clinical data--> | tradename =...") |
m (Protected "Terguride": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (One intermediate revision by one other user not shown) | |||
| Line 35: | Line 35: | ||
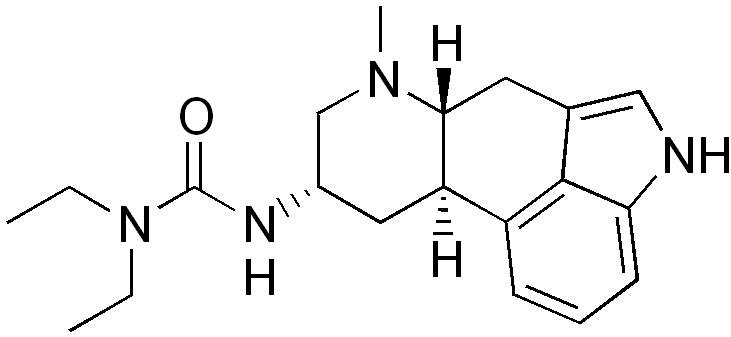
| smiles = CCN(CC)C(=O)N[C@H]1C[C@H]2[C@@H](CC3=CNC4=CC=CC2=C34)N(C1)C | | smiles = CCN(CC)C(=O)N[C@H]1C[C@H]2[C@@H](CC3=CNC4=CC=CC2=C34)N(C1)C | ||
}} | }} | ||
__NOTOC__ | |||
{{SI}} | |||
{{CMG}} | |||
==Overview== | |||
'''Terguride''' ([[International Nonproprietary Name|INN]]) is a [[serotonin]] [[antagonist]]. It is used for the treatment of hyperprolactinemia. Terguride is an oral, potent antagonist of 5-HT2B and 5-HT2A (serotonin) receptors. Serotonin stimulates the proliferation of pulmonary artery smooth muscle cells, and induces fibrosis in the wall of pulmonary arteries. Together, this causes vascular remodeling and narrowing of the pulmonary arteries. These changes result in increased vascular resistance and PAH. Due to the potential anti-proliferative and anti-fibrotic activity of terguride, this potential medicine could offer the hope of achieving reversal of pulmonary artery vascular remodeling and attenuation of disease progression.<ref>Janssen W, Schymura Y, Novoyatleva T, Kojonazarov B, Boehm M, Wietelmann A, Luitel H, Murmann K, Krompiec DR, Tretyn A, Pullamsetti SS, Weissmann N, Seeger W, Ghofrani HA, Schermuly RT. 5-HT2B Receptor Antagonists Inhibit Fibrosis and Protect from RV Heart Failure. ''Biomed Research International''. 2015;2015:438403. doi 10.1155/2015/438403 PMID 25667920</ref> | '''Terguride''' ([[International Nonproprietary Name|INN]]) is a [[serotonin]] [[antagonist]]. It is used for the treatment of hyperprolactinemia. Terguride is an oral, potent antagonist of 5-HT2B and 5-HT2A (serotonin) receptors. Serotonin stimulates the proliferation of pulmonary artery smooth muscle cells, and induces fibrosis in the wall of pulmonary arteries. Together, this causes vascular remodeling and narrowing of the pulmonary arteries. These changes result in increased vascular resistance and PAH. Due to the potential anti-proliferative and anti-fibrotic activity of terguride, this potential medicine could offer the hope of achieving reversal of pulmonary artery vascular remodeling and attenuation of disease progression.<ref>Janssen W, Schymura Y, Novoyatleva T, Kojonazarov B, Boehm M, Wietelmann A, Luitel H, Murmann K, Krompiec DR, Tretyn A, Pullamsetti SS, Weissmann N, Seeger W, Ghofrani HA, Schermuly RT. 5-HT2B Receptor Antagonists Inhibit Fibrosis and Protect from RV Heart Failure. ''Biomed Research International''. 2015;2015:438403. doi 10.1155/2015/438403 PMID 25667920</ref> | ||
In May 2008, terguride was granted [[orphan drug]] status for the treatment of [[pulmonary arterial hypertension]].<ref>[http://www.presseportal.ch/de/pm/100015139/100561578/ergonex_pharma_gmbh Presseportal (Swiss press portal, in German)]</ref> | In May 2008, terguride was granted [[orphan drug]] status for the treatment of [[pulmonary arterial hypertension]].<ref>[http://www.presseportal.ch/de/pm/100015139/100561578/ergonex_pharma_gmbh Presseportal (Swiss press portal, in German)]</ref> | ||
In May 2010 | In May 2010 Pfizer purchased world-wide rights for the drug.<ref>[http://www.theday.com/article/20100513/BIZ02/305139376/1044 TheDay.com 5/10/2010]</ref> | ||
== References == | == References == | ||
{{Reflist}} | {{Reflist|2}} | ||
[[Category:Dopamine agonists]] | [[Category:Dopamine agonists]] | ||
[[Category:Orphan drugs]] | [[Category:Orphan drugs]] | ||
[[Category:Ureas]] | [[Category:Ureas]] | ||
[[Category:Drug]] | |||
Latest revision as of 17:14, 20 August 2015
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| UNII | |
| KEGG | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C20H28N4O |
| Molar mass | 340.46 g/mol |
| 3D model (JSmol) | |
| |
| (verify) | |
|
WikiDoc Resources for Terguride |
|
Articles |
|---|
|
Most recent articles on Terguride |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Terguride at Clinical Trials.gov Clinical Trials on Terguride at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Terguride
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Terguride Discussion groups on Terguride Directions to Hospitals Treating Terguride Risk calculators and risk factors for Terguride
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Terguride |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Terguride (INN) is a serotonin antagonist. It is used for the treatment of hyperprolactinemia. Terguride is an oral, potent antagonist of 5-HT2B and 5-HT2A (serotonin) receptors. Serotonin stimulates the proliferation of pulmonary artery smooth muscle cells, and induces fibrosis in the wall of pulmonary arteries. Together, this causes vascular remodeling and narrowing of the pulmonary arteries. These changes result in increased vascular resistance and PAH. Due to the potential anti-proliferative and anti-fibrotic activity of terguride, this potential medicine could offer the hope of achieving reversal of pulmonary artery vascular remodeling and attenuation of disease progression.[1]
In May 2008, terguride was granted orphan drug status for the treatment of pulmonary arterial hypertension.[2] In May 2010 Pfizer purchased world-wide rights for the drug.[3]
References
- ↑ Janssen W, Schymura Y, Novoyatleva T, Kojonazarov B, Boehm M, Wietelmann A, Luitel H, Murmann K, Krompiec DR, Tretyn A, Pullamsetti SS, Weissmann N, Seeger W, Ghofrani HA, Schermuly RT. 5-HT2B Receptor Antagonists Inhibit Fibrosis and Protect from RV Heart Failure. Biomed Research International. 2015;2015:438403. doi 10.1155/2015/438403 PMID 25667920
- ↑ Presseportal (Swiss press portal, in German)
- ↑ TheDay.com 5/10/2010
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without InChI source
- Dopamine agonists
- Orphan drugs
- Ureas
- Drug