Suxamethonium chloride: Difference between revisions
No edit summary |
No edit summary |
||
| (19 intermediate revisions by the same user not shown) | |||
| Line 7: | Line 7: | ||
|hasBlackBoxWarning=Yes | |hasBlackBoxWarning=Yes | ||
|adverseReactions=[[ophthalmic]]: raised intraocular pressure | |adverseReactions=[[ophthalmic]]: raised intraocular pressure | ||
|blackBoxWarningTitle=<span style="color:#FF0000;"> | |blackBoxWarningTitle=<span style="color:#FF0000;">Risk Of Cardiac Arrest From Hyperkalemic Rhabdomyolysis</span> | ||
|blackBoxWarningBody=<i><span style="color:#FF0000;"></span></i> There have been rare reports of acute rhabdomyolysis with hyperkalemia followed by ventricular dysrhythmias, cardiac arrest, and death after the administration of succinylcholine to apparently healthy children who were subsequently found to have undiagnosed skeletal muscle myopathy, most frequently Duchenne's muscular dystrophy. | |blackBoxWarningBody=<i><span style="color:#FF0000;"></span></i> | ||
* There have been rare reports of acute rhabdomyolysis with hyperkalemia followed by ventricular dysrhythmias, cardiac arrest, and death after the administration of succinylcholine to apparently healthy children who were subsequently found to have undiagnosed skeletal muscle myopathy, most frequently Duchenne's muscular dystrophy. | |||
* This syndrome often presents as peaked T-waves and sudden cardiac arrest within minutes after the administration of the drug in healthy appearing children (usually, but not exclusively, males, and most frequently 8 years of age or younger). There have also been reports in adolescents. | |||
* Therefore, when a healthy appearing infant or child develops cardiac arrest soon after administration of succinylcholine not felt to be due to inadequate ventilation, oxygenation, or anesthetic overdose, immediate treatment for hyperkalemia should be instituted. This should include administration of intravenous calcium, bicarbonate, and glucose with insulin, with hyperventilation. Due to the abrupt onset of this syndrome, routine resuscitative measures are likely to be unsuccessful. However, extraordinary and prolonged resuscitative efforts have resulted in successful resuscitation in some reported cases. In addition, in the presence of signs of malignant hyperthermia, appropriate treatment should be instituted concurrently. | |||
* Since there may be no signs or symptoms to alert the practitioner to which patients are at risk, it is recommended that the use of succinylcholine in children should be reserved for emergency intubation or instances where immediate securing of the airway is necessary, e.g. laryngospasm, difficult airway, full stomach, or for intramuscular use when a suitable vein is inaccessible (see Precautions: Pediatric Use and Dosage and administration). | |||
|fdaLIADAdult=====Induction of neuromuscular blockade, Adjunct to general anesthesia, to facilitate endotracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation==== | |||
* Short procedures | |||
:* 0.6 mg/kg IV (range 0.3 to 1.1 mg/kg) over 10 to 30 seconds | |||
* Long procedures | |||
:* 2.5 to 4.3 mg/min continuous IV infusion | |||
* Long procedures | |||
:* 0.3 to 1.1 mg/kg IV initially followed by 0.04 to 0.07 mg/kg at appropriate intervals | |||
* If suitable vein is inaccessible | |||
:* 3 to 4 mg/kg IM,; MAX 150 mg | |||
* Rapid sequence intubation | |||
:* 1.5 mg/kg IV push | |||
|offLabelAdultGuideSupport=* Electroconvulsive therapy; Adjunct | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Suxamethonium chloride in adult patients. | |||
|fdaLIADPed=* Due to risk of cardiac arrest from hyperkalemic rhabdomyolysis, use in children should be reserved for emergency intubation or instances where immediate securing of the airway is necessary. | |||
====Induction of neuromuscular blockade, Adjunct to general anesthesia, to facilitate endotracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation==== | |||
* Emergency tracheal intubation, infants and small pediatric patients | |||
:* 2 mg/kg IV | |||
* Emergency tracheal intubation, older pediatric patients and adolescents | |||
:* 1 mg/kg | |||
* If suitable vein is inaccessible | |||
:*3 to 4 mg/kg IM, MAX 150 mg | |||
* Rapid sequence intubation: older children and adolescents | |||
:* 1 mg/kg IV | |||
* Rapid sequence intubation: infants and small children | |||
:* 2 mg/kg IV | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Suxamethonium chloride in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Suxamethonium chloride in pediatric patients. | |||
|contraindications=* Succinylcholine is contraindicated in persons with personal or familial history of [[malignant hyperthermia]], [[skeletal muscle myopathies]], and known hypersensitivity to the drug. It is also contraindicated in patients after the acute phase of injury following major burns, multiple trauma, extensive denervation of skeletal muscle, or upper motor neuron injury, because succinylcholine administered to such individuals may result in severe [[hyperkalemia]] which may result in [[cardiac arrest]] (see Warnings). The risk of hyperkalemia in these patients increases over time and usually peaks at 7 to 10 days after the injury. The risk is dependent on the extent and location of the injury. The precise time of onset and the duration of the risk period are not known. | |||
|warnings=* Succinylcholine should be used only by those skilled in the management of artificial respiration and only when facilities are instantly available for tracheal intubation and for providing adequate ventilation of the patient, including the administration of oxygen under positive pressure and the elimination of carbon dioxide. The clinician must be prepared to assist or control respiration. | |||
* To avoid distress to the patient, succinylcholine should not be administered before unconsciousness has been induced. In emergency situations, however, it may be necessary to administer succinylcholine before unconsciousness is induced. | |||
* Succinylcholine is metabolized by plasma cholinesterase and should be used with caution, if at all, in patients known to be or suspected of being homozygous for the atypical plasma cholinesterase gene. | |||
====Anaphylaxis==== | |||
* Severe anaphylactic reactions to neuromuscular blocking agents, including Succinylcholine, have been reported. These reactions have in some cases been life-threatening and fatal. Due to the potential severity of these reactions, the necessary precautions, such as the immediate availability of appropriate emergency treatment, should be taken. Precautions should also be taken in those individuals who have had previous anaphylactic reactions to other neuromuscular blocking agents since cross-reactivity between neuromuscular blocking agents, both depolarizing and non-depolarizing, has been reported in this class of drugs. | |||
====Hyperkalemia==== | |||
* (See box warning.) Succinylcholine should be administered with GREAT CAUTION to patients suffering from electrolyte abnormalities and those who may have massive digitalis toxicity, because in these circumstances succinylcholine may induce serious cardiac arrhythmias or cardiac arrest due to hyperkalemia. | |||
* Great caution should be observed if succinylcholine is administered to patients during the acute phase of injury following major burns, multiple trauma, extensive denervation of skeletal muscle, or upper motor neuron injury (see Contraindications). The risk of hyperkalemia in these patients increases over time and usually peaks at 7 to 10 days after the injury. The risk is dependent on the extent and location of the injury. The precise time of onset and the duration of the risk period are undetermined. Patients with chronic abdominal infection, subarachnoid hemorrhage, or conditions causing degeneration of central and peripheral nervous systems should receive succinylcholine with Great caution because of the potential for developing severe hyperkalemia. | |||
====Malignant Hyperthermia==== | |||
* Succinylcholine administration has been associated with acute onset of [[malignant hyperthermia]], a potentially fatal hypermetabolic state of skeletal muscle. The risk of developing malignant hyperthermia following succinylcholine administration increases with the concomitant administration of volatile anesthetics. Malignant hyperthermia frequently presents as intractable spasm of the jaw muscles (masseter spasm) which may progress to generalized rigidity, increased oxygen demand, [[tachycardia]], [[tachypnea]], and profound [[hyperpyrexia]]. Successful outcome depends on recognition of early signs, such as jaw muscle spasm, [[acidosis]], or generalized rigidity to initial administration of succinylcholine for tracheal intubation, or failure of tachycardia to respond to deepening anesthesia. Skin mottling, rising temperature, and coagulopathies may occur later in the course of the hypermetabolic process. Recognition of the syndrome is a signal for discontinuance of anesthesia, attention to increased oxygen consumption, correction of acidosis, support of circulation, assurance of adequate urinary output, and institution of measures to control rising temperature. Intravenous [[dantrolene sodium]] is recommended as an adjunct to supportive measures in the management of this problem. Consult literature references and the dantrolene prescribing information for additional information about the management of malignant hyperthermic crisis. Continuous monitoring of temperature and expired CO2 is recommended as an aid to early recognition of malignant hyperthermia. | |||
====Other==== | |||
* In both adults and children, the incidence of bradycardia, which may progress to [[asystole]], is higher following a second dose of [[succinylcholine]]. The incidence and severity of bradycardia is higher in children than in adults. Pretreatment with anticholinergic agents (e.g., [[atropine]]) may reduce the occurrence of [[bradyarrhythmias]]. | |||
* Succinylcholine causes an increase in intraocular pressure. It should not be used in instances in which an increase in intraocular pressure is undesirable (e.g., [[narrow angle glaucoma]], penetrating eye injury) unless the potential benefit of its use outweighs the potential risk. | |||
* Succinylcholine is acidic (pH = 3.5) and should not be mixed with alkaline solutions having a pH greater than 8.5 (e.g., barbiturate solutions). | |||
|clinicalTrials=* Adverse reactions to succinylcholine consist primarily of an extension of its pharmacological actions. Succinylcholine causes profound muscle relaxation resulting in respiratory depression to the point of apnea; this effect may be prolonged. Hypersensitivity reactions, including anaphylaxis, may occur in rare instances. The following additional adverse reactions have been reported: [[cardiac arrest]], [[malignant hyperthermia]], [[arrhythmias]], [[bradycardia]], [[tachycardia]], [[hypertension]], [[hypotension]], [[hyperkalemia]], prolonged respiratory depression or apnea, increased intraocular pressure, [[muscle fasciculation]], [[jaw rigidity]], postoperative muscle pain, [[rhabdomyolysis]] with possible [[myoglobinuric]] [[acute renal failur]]e, excessive salivation, and rash. | |||
* There have been post-marketing reports of severe allergic reactions ([[anaphylactic]] and anaphylactoid reactions) associated with use of neuromuscular blocking agents, including Succinylcholine. These reactions, in some cases, have been life-threatening and fatal. Because these reactions were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency (see Warnings and Precautions). | |||
|drugInteractions=* Drugs which may enhance the neuromuscular blocking action of [[succinylcholine]] include: [[promazine]], [[oxytocin]], [[aprotinin]], certain non-penicillin antibiotics, [[quinidine]], β-adrenergic blockers, [[procainamide]], [[lidocaine]], [[trimethaphan]], [[lithium carbonate]], magnesium salts, [[quinine]], [[chloroquine]], [[diethylether]], [[isoflurane]], [[desflurane]], [[metoclopramide]], and [[terbutaline]]. The neuromuscular blocking effect of succinylcholine may be enhanced by drugs that reduce plasma cholinesterase activity (e.g., chronically administered oral contraceptives, [[glucocorticoids]], or certain monoamine oxidase inhibitors) or by drugs that irreversibly inhibit plasma cholinesterase (see Precautions). | |||
* If other neuromuscular blocking agents are to be used during the same procedure, the possibility of a synergistic or antagonistic effect should be considered. | |||
|FDAPregCat=C | |||
|useInPregnancyFDA= | |||
====Teratogenic Effects==== | |||
* Animal reproduction studies have not been conducted with succinylcholine chloride. It is also not known whether succinylcholine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Succinylcholine should be given to a pregnant woman only if clearly needed. | |||
====Nonteratogenic Effects==== | |||
* Plasma cholinesterase levels are decreased by approximately 24% during pregnancy and for several days postpartum. Therefore, a higher proportion of patients may be expected to show increased sensitivity (prolonged apnea) to succinylcholine when pregnant than when nonpregnant. | |||
|useInLaborDelivery=* Succinylcholine is commonly used to provide muscle relaxation during delivery by cesarean section. While small amounts of succinylcholine are known to cross the placental barrier, under normal conditions the quantity of drug that enters fetal circulation after a single dose of 1 mg/kg to the mother should not endanger the fetus. However, since the amount of drug that crosses the placental barrier is dependent on the concentration gradient between the maternal and fetal circulations, residual neuromuscular blockade (apnea and flaccidity) may occur in the neonate after repeated high doses to, or in the presence of atypical plasma cholinesterase in, the mother. | |||
|useInNursing=* It is not known whether succinylcholine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised following succinylcholine administration to a nursing woman. | |||
|useInPed=* There are rare reports of [[ventricular dysrhythmias]] and [[cardiac arrest]] secondary to acute [[rhabdomyolysis]] with [[hyperkalemia]] in apparently healthy children who receive succinylcholine (see Box warning). Many of these children were subsequently found to have a skeletal muscle myopathy such as [[Duchenne’s muscular dystrophy]] whose clinical signs were not obvious. The syndrome often presents as sudden cardiac arrest within minutes after the administration of succinylcholine. These children are usually, but not exclusively, males, and most frequently 8 years of age or younger. There have also been reports in adolescents. There may be no signs or symptoms to alert the practitioner to which patients are at risk. A careful history and physical may identify developmental delays suggestive of a [[myopathy]]. A preoperative [[creatine kinase]] could identify some but not all patients at risk. Due to the abrupt onset of this syndrome, routine resuscitative measures are likely to be unsuccessful. Careful monitoring of the electrocardiogram may alert the practitioner to peaked T-waves (an early sign). Administration of IV [[calcium, bicarbonate]], and [[glucose]] with [[insulin]], with hyperventilation have resulted in successful resuscitation in some of the reported cases. Extraordinary and prolonged resuscitative efforts have been effective in some cases. In addition, in the presence of signs of [[malignant hyperthermia]], appropriate treatment should be initiated concurrently (see Warnings). Since it is difficult to identify which patients are at risk, it is recommended that the use of succinylcholine in children should be reserved for emergency intubation or instances where immediate securing of the airway is necessary, e.g., [[laryngospasm]], difficult airway, full stomach, or for intramuscular use when a suitable vein is inaccessible. | |||
* As in adults, the incidence of bradycardia in children is higher following the second dose of succinylcholine. The incidence and severity of bradycardia is higher in children than in adults. Pretreatment with anticholinergic agents, e.g., atropine, may reduce the occurrence of bradyarrhythmias. | |||
|drugBox={{drugbox2 | |||
| Verifiedfields = changed | |||
| verifiedrevid = 477162668 | |||
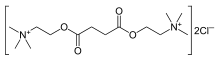
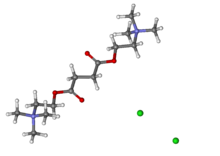
| IUPAC_name = 2,2'-[(1,4-dioxobutane-1,4-diyl)bis(oxy)]bis<br>(''N'',''N'',''N''-trimethylethanaminium) | |||
| image = Succunylcholine wiki1.svg.png | |||
| image2 = Succinyl choline wiki2.png | |||
| drug_name = Succinylcholine | |||
<!--Clinical data--> | |||
| tradename = Quelicin | |||
| Drugs.com = {{drugs.com|monograph|succinylcholine-chloride}} | |||
| pregnancy_AU = A | |||
| pregnancy_US = C | |||
| legal_AU = S4 | |||
| legal_UK = POM | |||
| legal_US = Rx-only | |||
| routes_of_administration = [[Intravenous therapy|Intravenous]], [[Intramuscular]] | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = NA | |||
| protein_bound = | |||
| metabolism = {{nowrap|By [[pseudocholinesterase]],}} to [[succinylmonocholine]] and [[choline]] | |||
| elimination_half-life = | |||
| excretion = [[Kidney|Renal]] (10%) | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 306-40-1 | |||
| ATC_prefix = M03 | |||
| ATC_suffix = AB01 | |||
| ATC_supplemental = | |||
| PubChem = 22475 | |||
| IUPHAR_ligand = 4004 | |||
| DrugBank_Ref = {{drugbankcite|changed|drugbank}} | |||
| DrugBank = DB00202 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 21080 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = J2R869A8YF | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00766 | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 61219 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 983 | |||
<!--Chemical data--> | |||
| | | C=14 | H=30 | N=2 | O=4 | ||
| molecular_weight = 290.399 g/mol | |||
| smiles = [Cl-].[Cl-].O=C(OCC[N+](C)(C)C)CCC(=O)OCC[N+](C)(C)C | |||
| InChI = 1/C14H30N2O4.2ClH/c1-15(2,3)9-11-19-13(17)7-8-14(18)20-12-10-16(4,5)6;;/h7-12H2,1-6H3;2*1H/q+2;;/p-2 | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | | StdInChI = 1S/C14H30N2O4.2ClH/c1-15(2,3)9-11-19-13(17)7-8-14(18)20-12-10-16(4,5)6;;/h7-12H2,1-6H3;2*1H/q+2;;/p-2 | ||
| | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | | StdInChIKey = YOEWQQVKRJEPAE-UHFFFAOYSA-L | ||
}} | |||
|mechAction=* Succinylcholine is a depolarizing [[skeletal muscle relaxant]]. As does [[acetylcholine]], it combines with the cholinergic receptors of the motor end plate to produce depolarization. This depolarization may be observed as [[fasciculations]]. Subsequent neuromuscular transmission is inhibited so long as adequate concentration of succinylcholine remains at the receptor site. Onset of flaccid paralysis is rapid (less than 1 minute after IV administration), and with single administration lasts approximately 4 to 6 minutes. | |||
|structure=* Succinylcholine (succinylcholine chloride) is an ultra short-acting depolarizing-type, skeletal muscle relaxant for intravenous (IV) administration. | |||
* Succinylcholine chloride is a white, odorless, slightly bitter powder and very soluble in water. The drug is unstable in alkaline solutions but relatively stable in acid solutions, depending upon the concentration of the solution and the storage temperature. Solutions of succinylcholine chloride should be stored under refrigeration to preserve potency. Succinylcholine Injection is a sterile nonpyrogenic solution for IV injection, containing 20 mg succinylcholine chloride in each mL and made isotonic with sodium chloride. The pH is adjusted to 3.5 with hydrochloric acid. Methylparaben (0.1%) is added as a preservative. | |||
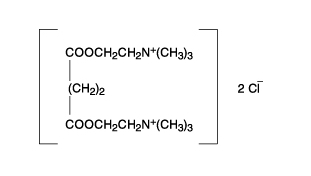
The chemical name for succinylcholine chloride is 2,2'-[(1,4-dioxo-1,4-butanediyl)bis(oxy)]bis[N,N,N-trimethylethanaminium] dichloride, and the structural formula is: | |||
| | [[File:SUCCINYLCHOLINEstructure .jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | ||
|PD=* Succinylcholine has no direct effect on the [[myocardium]]. Succinylcholine stimulates both autonomic ganglia and muscarinic receptors which may cause changes in cardiac rhythm, including [[cardiac arrest]]. Changes in rhythm, including cardiac arrest, may also result from vagal stimulation, which may occur during surgical procedures, or from hyperkalemia, particularly in children (see Precautions: Pediatric Use). These effects are enhanced by halogenated anesthetics. | |||
* Succinylcholine causes an increase in intraocular pressure immediately after its injection and during the fasciculation phase, and slight increases which may persist after onset of complete paralysis (see Warnings). | |||
* Succinylcholine may cause slight increases in intracranial pressure immediately after its injection and during the fasciculation phase (see PRECAUTIONS). | |||
* As with other neuromuscular blocking agents, the potential for releasing histamine is present following succinylcholine administration. Signs and symptoms of histamine-mediated release such as [[flushing]], [[hypotension]], and [[bronchoconstriction]] are, however, uncommon in normal clinical usage. | |||
* Succinylcholine has no effect on consciousness, pain threshold, or cerebration. It should be used only with adequate anesthesia (see WARNINGS). | |||
|PK=* Succinylcholine is rapidly hydrolyzed by plasma cholinesterase to succinylmonocholine (which possesses clinically insignificant depolarizing muscle relaxant properties) and then more slowly to succinic acid and choline (see Precautions). About 10% of the drug is excreted unchanged in the urine. The paralysis following administration of succinylcholine is progressive, with differing sensitivities of different muscles. This initially involves consecutively the levator muscles of the face, muscles of the glottis, and finally, the intercostals and the diaphragm and all other skeletal muscles. | |||
* Succinylcholine has no direct action on the uterus or other smooth muscle structures. Because it is highly ionized and has low fat solubility, it does not readily cross the placenta. | |||
* Tachyphylaxis occurs with repeated administration (see Precautions). | |||
* Depending on the dose and duration of succinylcholine administration, the characteristic depolarizing neuromuscular block (Phase I block) may change to a block with characteristics superficially resembling a nondepolarizing block (Phase II block). This may be associated with prolonged respiratory muscle paralysis or weakness in patients who manifest the transition to Phase II block. When this diagnosis is confirmed by peripheral nerve stimulation, it may sometimes be reversed with anticholinesterase drugs such as [[neostigmine]] (see Precautions). Anticholinesterase drugs may not always be effective. If given before succinylcholine is metabolized by cholinesterase, anticholinesterase drugs may prolong rather than shorten paralysis. | |||
|howSupplied=* For immediate injection of single doses for short procedures: Succinylcholine (succinylcholine chloride) Injection, 20 mg in each mL. | |||
: Multiple-dose vials of 10 mL, box of 10 vials (NDC 0781-3009-95). | |||
|storage=* Store in refrigerator at 2° to 8°C (36° to 46°F). The multi-dose vials are stable for up to 14 days at room temperature without significant loss of potency. | |||
: Manufactured by | |||
: Agila Specialties Pvt. Ltd. | |||
: (Specialty Formulation Facility) | |||
: Bangalore – 560 105, India for | |||
: Sandoz Inc. | |||
: Princeton, NJ 08540 | |||
: 04-2011 | |||
: 1016206 | |||
: Manufactured by | |||
: Agila Specialties Pvt. Ltd. | |||
: (Sterile Product Division) | |||
: Bangalore – 560 076, India for | |||
: Sandoz Inc. | |||
: Princeton, NJ 08540 | |||
: 04-2011 | |||
: 1016227 | |||
: 200 mg Label | |||
|alcohol=Alcohol-Suxamethonium chloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Suxamethonium chloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | |||
{{PillImage}} | |||
{{LabelImage | |||
|fileName=Succinyl choline label.png | |||
}} | }} | ||
Latest revision as of 15:30, 25 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Risk Of Cardiac Arrest From Hyperkalemic Rhabdomyolysis
See full prescribing information for complete Boxed Warning.
|
Overview
Suxamethonium chloride is a skeletal muscle relaxant, neuromuscular blocking drug that is FDA approved for the {{{indicationType}}} of induction of neuromuscular blockade, adjunct to general anesthesia, to facilitate endotracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation, rapid sequence intubation. There is a Black Box Warning for this drug as shown here. Common adverse reactions include ophthalmic: raised intraocular pressure.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Induction of neuromuscular blockade, Adjunct to general anesthesia, to facilitate endotracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation
- Short procedures
- 0.6 mg/kg IV (range 0.3 to 1.1 mg/kg) over 10 to 30 seconds
- Long procedures
- 2.5 to 4.3 mg/min continuous IV infusion
- Long procedures
- 0.3 to 1.1 mg/kg IV initially followed by 0.04 to 0.07 mg/kg at appropriate intervals
- If suitable vein is inaccessible
- 3 to 4 mg/kg IM,; MAX 150 mg
- Rapid sequence intubation
- 1.5 mg/kg IV push
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- Electroconvulsive therapy; Adjunct
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Suxamethonium chloride in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Due to risk of cardiac arrest from hyperkalemic rhabdomyolysis, use in children should be reserved for emergency intubation or instances where immediate securing of the airway is necessary.
Induction of neuromuscular blockade, Adjunct to general anesthesia, to facilitate endotracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation
- Emergency tracheal intubation, infants and small pediatric patients
- 2 mg/kg IV
- Emergency tracheal intubation, older pediatric patients and adolescents
- 1 mg/kg
- If suitable vein is inaccessible
- 3 to 4 mg/kg IM, MAX 150 mg
- Rapid sequence intubation: older children and adolescents
- 1 mg/kg IV
- Rapid sequence intubation: infants and small children
- 2 mg/kg IV
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Suxamethonium chloride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Suxamethonium chloride in pediatric patients.
Contraindications
- Succinylcholine is contraindicated in persons with personal or familial history of malignant hyperthermia, skeletal muscle myopathies, and known hypersensitivity to the drug. It is also contraindicated in patients after the acute phase of injury following major burns, multiple trauma, extensive denervation of skeletal muscle, or upper motor neuron injury, because succinylcholine administered to such individuals may result in severe hyperkalemia which may result in cardiac arrest (see Warnings). The risk of hyperkalemia in these patients increases over time and usually peaks at 7 to 10 days after the injury. The risk is dependent on the extent and location of the injury. The precise time of onset and the duration of the risk period are not known.
Warnings
|
Risk Of Cardiac Arrest From Hyperkalemic Rhabdomyolysis
See full prescribing information for complete Boxed Warning.
|
- Succinylcholine should be used only by those skilled in the management of artificial respiration and only when facilities are instantly available for tracheal intubation and for providing adequate ventilation of the patient, including the administration of oxygen under positive pressure and the elimination of carbon dioxide. The clinician must be prepared to assist or control respiration.
- To avoid distress to the patient, succinylcholine should not be administered before unconsciousness has been induced. In emergency situations, however, it may be necessary to administer succinylcholine before unconsciousness is induced.
- Succinylcholine is metabolized by plasma cholinesterase and should be used with caution, if at all, in patients known to be or suspected of being homozygous for the atypical plasma cholinesterase gene.
Anaphylaxis
- Severe anaphylactic reactions to neuromuscular blocking agents, including Succinylcholine, have been reported. These reactions have in some cases been life-threatening and fatal. Due to the potential severity of these reactions, the necessary precautions, such as the immediate availability of appropriate emergency treatment, should be taken. Precautions should also be taken in those individuals who have had previous anaphylactic reactions to other neuromuscular blocking agents since cross-reactivity between neuromuscular blocking agents, both depolarizing and non-depolarizing, has been reported in this class of drugs.
Hyperkalemia
- (See box warning.) Succinylcholine should be administered with GREAT CAUTION to patients suffering from electrolyte abnormalities and those who may have massive digitalis toxicity, because in these circumstances succinylcholine may induce serious cardiac arrhythmias or cardiac arrest due to hyperkalemia.
- Great caution should be observed if succinylcholine is administered to patients during the acute phase of injury following major burns, multiple trauma, extensive denervation of skeletal muscle, or upper motor neuron injury (see Contraindications). The risk of hyperkalemia in these patients increases over time and usually peaks at 7 to 10 days after the injury. The risk is dependent on the extent and location of the injury. The precise time of onset and the duration of the risk period are undetermined. Patients with chronic abdominal infection, subarachnoid hemorrhage, or conditions causing degeneration of central and peripheral nervous systems should receive succinylcholine with Great caution because of the potential for developing severe hyperkalemia.
Malignant Hyperthermia
- Succinylcholine administration has been associated with acute onset of malignant hyperthermia, a potentially fatal hypermetabolic state of skeletal muscle. The risk of developing malignant hyperthermia following succinylcholine administration increases with the concomitant administration of volatile anesthetics. Malignant hyperthermia frequently presents as intractable spasm of the jaw muscles (masseter spasm) which may progress to generalized rigidity, increased oxygen demand, tachycardia, tachypnea, and profound hyperpyrexia. Successful outcome depends on recognition of early signs, such as jaw muscle spasm, acidosis, or generalized rigidity to initial administration of succinylcholine for tracheal intubation, or failure of tachycardia to respond to deepening anesthesia. Skin mottling, rising temperature, and coagulopathies may occur later in the course of the hypermetabolic process. Recognition of the syndrome is a signal for discontinuance of anesthesia, attention to increased oxygen consumption, correction of acidosis, support of circulation, assurance of adequate urinary output, and institution of measures to control rising temperature. Intravenous dantrolene sodium is recommended as an adjunct to supportive measures in the management of this problem. Consult literature references and the dantrolene prescribing information for additional information about the management of malignant hyperthermic crisis. Continuous monitoring of temperature and expired CO2 is recommended as an aid to early recognition of malignant hyperthermia.
Other
- In both adults and children, the incidence of bradycardia, which may progress to asystole, is higher following a second dose of succinylcholine. The incidence and severity of bradycardia is higher in children than in adults. Pretreatment with anticholinergic agents (e.g., atropine) may reduce the occurrence of bradyarrhythmias.
- Succinylcholine causes an increase in intraocular pressure. It should not be used in instances in which an increase in intraocular pressure is undesirable (e.g., narrow angle glaucoma, penetrating eye injury) unless the potential benefit of its use outweighs the potential risk.
- Succinylcholine is acidic (pH = 3.5) and should not be mixed with alkaline solutions having a pH greater than 8.5 (e.g., barbiturate solutions).
Adverse Reactions
Clinical Trials Experience
- Adverse reactions to succinylcholine consist primarily of an extension of its pharmacological actions. Succinylcholine causes profound muscle relaxation resulting in respiratory depression to the point of apnea; this effect may be prolonged. Hypersensitivity reactions, including anaphylaxis, may occur in rare instances. The following additional adverse reactions have been reported: cardiac arrest, malignant hyperthermia, arrhythmias, bradycardia, tachycardia, hypertension, hypotension, hyperkalemia, prolonged respiratory depression or apnea, increased intraocular pressure, muscle fasciculation, jaw rigidity, postoperative muscle pain, rhabdomyolysis with possible myoglobinuric acute renal failure, excessive salivation, and rash.
- There have been post-marketing reports of severe allergic reactions (anaphylactic and anaphylactoid reactions) associated with use of neuromuscular blocking agents, including Succinylcholine. These reactions, in some cases, have been life-threatening and fatal. Because these reactions were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency (see Warnings and Precautions).
Postmarketing Experience
There is limited information regarding Suxamethonium chloride Postmarketing Experience in the drug label.
Drug Interactions
- Drugs which may enhance the neuromuscular blocking action of succinylcholine include: promazine, oxytocin, aprotinin, certain non-penicillin antibiotics, quinidine, β-adrenergic blockers, procainamide, lidocaine, trimethaphan, lithium carbonate, magnesium salts, quinine, chloroquine, diethylether, isoflurane, desflurane, metoclopramide, and terbutaline. The neuromuscular blocking effect of succinylcholine may be enhanced by drugs that reduce plasma cholinesterase activity (e.g., chronically administered oral contraceptives, glucocorticoids, or certain monoamine oxidase inhibitors) or by drugs that irreversibly inhibit plasma cholinesterase (see Precautions).
- If other neuromuscular blocking agents are to be used during the same procedure, the possibility of a synergistic or antagonistic effect should be considered.
Use in Specific Populations
Pregnancy
Teratogenic Effects
- Animal reproduction studies have not been conducted with succinylcholine chloride. It is also not known whether succinylcholine can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Succinylcholine should be given to a pregnant woman only if clearly needed.
Nonteratogenic Effects
- Plasma cholinesterase levels are decreased by approximately 24% during pregnancy and for several days postpartum. Therefore, a higher proportion of patients may be expected to show increased sensitivity (prolonged apnea) to succinylcholine when pregnant than when nonpregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Suxamethonium chloride in women who are pregnant.
Labor and Delivery
- Succinylcholine is commonly used to provide muscle relaxation during delivery by cesarean section. While small amounts of succinylcholine are known to cross the placental barrier, under normal conditions the quantity of drug that enters fetal circulation after a single dose of 1 mg/kg to the mother should not endanger the fetus. However, since the amount of drug that crosses the placental barrier is dependent on the concentration gradient between the maternal and fetal circulations, residual neuromuscular blockade (apnea and flaccidity) may occur in the neonate after repeated high doses to, or in the presence of atypical plasma cholinesterase in, the mother.
Nursing Mothers
- It is not known whether succinylcholine is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised following succinylcholine administration to a nursing woman.
Pediatric Use
- There are rare reports of ventricular dysrhythmias and cardiac arrest secondary to acute rhabdomyolysis with hyperkalemia in apparently healthy children who receive succinylcholine (see Box warning). Many of these children were subsequently found to have a skeletal muscle myopathy such as Duchenne’s muscular dystrophy whose clinical signs were not obvious. The syndrome often presents as sudden cardiac arrest within minutes after the administration of succinylcholine. These children are usually, but not exclusively, males, and most frequently 8 years of age or younger. There have also been reports in adolescents. There may be no signs or symptoms to alert the practitioner to which patients are at risk. A careful history and physical may identify developmental delays suggestive of a myopathy. A preoperative creatine kinase could identify some but not all patients at risk. Due to the abrupt onset of this syndrome, routine resuscitative measures are likely to be unsuccessful. Careful monitoring of the electrocardiogram may alert the practitioner to peaked T-waves (an early sign). Administration of IV calcium, bicarbonate, and glucose with insulin, with hyperventilation have resulted in successful resuscitation in some of the reported cases. Extraordinary and prolonged resuscitative efforts have been effective in some cases. In addition, in the presence of signs of malignant hyperthermia, appropriate treatment should be initiated concurrently (see Warnings). Since it is difficult to identify which patients are at risk, it is recommended that the use of succinylcholine in children should be reserved for emergency intubation or instances where immediate securing of the airway is necessary, e.g., laryngospasm, difficult airway, full stomach, or for intramuscular use when a suitable vein is inaccessible.
- As in adults, the incidence of bradycardia in children is higher following the second dose of succinylcholine. The incidence and severity of bradycardia is higher in children than in adults. Pretreatment with anticholinergic agents, e.g., atropine, may reduce the occurrence of bradyarrhythmias.
Geriatic Use
There is no FDA guidance on the use of Suxamethonium chloride in geriatric settings.
Gender
There is no FDA guidance on the use of Suxamethonium chloride with respect to specific gender populations.
Race
There is no FDA guidance on the use of Suxamethonium chloride with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Suxamethonium chloride in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Suxamethonium chloride in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Suxamethonium chloride in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Suxamethonium chloride in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Suxamethonium chloride Administration in the drug label.
Monitoring
There is limited information regarding Suxamethonium chloride Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Suxamethonium chloride and IV administrations.
Overdosage
There is limited information regarding Suxamethonium chloride overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
| |
| |
Succinylcholine
| |
| Systematic (IUPAC) name | |
| 2,2'-[(1,4-dioxobutane-1,4-diyl)bis(oxy)]bis (N,N,N-trimethylethanaminium) | |
| Identifiers | |
| CAS number | |
| ATC code | M03 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 290.399 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | NA |
| Metabolism | By pseudocholinesterase, to succinylmonocholine and choline |
| Half life | ? |
| Excretion | Renal (10%) |
| Therapeutic considerations | |
| Pregnancy cat. | |
| Legal status |
Prescription Only (S4)(AU) POM(UK) [[Prescription drug|Template:Unicode-only]](US) |
| Routes | Intravenous, Intramuscular |
Mechanism of Action
- Succinylcholine is a depolarizing skeletal muscle relaxant. As does acetylcholine, it combines with the cholinergic receptors of the motor end plate to produce depolarization. This depolarization may be observed as fasciculations. Subsequent neuromuscular transmission is inhibited so long as adequate concentration of succinylcholine remains at the receptor site. Onset of flaccid paralysis is rapid (less than 1 minute after IV administration), and with single administration lasts approximately 4 to 6 minutes.
Structure
- Succinylcholine (succinylcholine chloride) is an ultra short-acting depolarizing-type, skeletal muscle relaxant for intravenous (IV) administration.
- Succinylcholine chloride is a white, odorless, slightly bitter powder and very soluble in water. The drug is unstable in alkaline solutions but relatively stable in acid solutions, depending upon the concentration of the solution and the storage temperature. Solutions of succinylcholine chloride should be stored under refrigeration to preserve potency. Succinylcholine Injection is a sterile nonpyrogenic solution for IV injection, containing 20 mg succinylcholine chloride in each mL and made isotonic with sodium chloride. The pH is adjusted to 3.5 with hydrochloric acid. Methylparaben (0.1%) is added as a preservative.
The chemical name for succinylcholine chloride is 2,2'-[(1,4-dioxo-1,4-butanediyl)bis(oxy)]bis[N,N,N-trimethylethanaminium] dichloride, and the structural formula is:
Pharmacodynamics
- Succinylcholine has no direct effect on the myocardium. Succinylcholine stimulates both autonomic ganglia and muscarinic receptors which may cause changes in cardiac rhythm, including cardiac arrest. Changes in rhythm, including cardiac arrest, may also result from vagal stimulation, which may occur during surgical procedures, or from hyperkalemia, particularly in children (see Precautions: Pediatric Use). These effects are enhanced by halogenated anesthetics.
- Succinylcholine causes an increase in intraocular pressure immediately after its injection and during the fasciculation phase, and slight increases which may persist after onset of complete paralysis (see Warnings).
- Succinylcholine may cause slight increases in intracranial pressure immediately after its injection and during the fasciculation phase (see PRECAUTIONS).
- As with other neuromuscular blocking agents, the potential for releasing histamine is present following succinylcholine administration. Signs and symptoms of histamine-mediated release such as flushing, hypotension, and bronchoconstriction are, however, uncommon in normal clinical usage.
- Succinylcholine has no effect on consciousness, pain threshold, or cerebration. It should be used only with adequate anesthesia (see WARNINGS).
Pharmacokinetics
- Succinylcholine is rapidly hydrolyzed by plasma cholinesterase to succinylmonocholine (which possesses clinically insignificant depolarizing muscle relaxant properties) and then more slowly to succinic acid and choline (see Precautions). About 10% of the drug is excreted unchanged in the urine. The paralysis following administration of succinylcholine is progressive, with differing sensitivities of different muscles. This initially involves consecutively the levator muscles of the face, muscles of the glottis, and finally, the intercostals and the diaphragm and all other skeletal muscles.
- Succinylcholine has no direct action on the uterus or other smooth muscle structures. Because it is highly ionized and has low fat solubility, it does not readily cross the placenta.
- Tachyphylaxis occurs with repeated administration (see Precautions).
- Depending on the dose and duration of succinylcholine administration, the characteristic depolarizing neuromuscular block (Phase I block) may change to a block with characteristics superficially resembling a nondepolarizing block (Phase II block). This may be associated with prolonged respiratory muscle paralysis or weakness in patients who manifest the transition to Phase II block. When this diagnosis is confirmed by peripheral nerve stimulation, it may sometimes be reversed with anticholinesterase drugs such as neostigmine (see Precautions). Anticholinesterase drugs may not always be effective. If given before succinylcholine is metabolized by cholinesterase, anticholinesterase drugs may prolong rather than shorten paralysis.
Nonclinical Toxicology
There is limited information regarding Suxamethonium chloride Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Suxamethonium chloride Clinical Studies in the drug label.
How Supplied
- For immediate injection of single doses for short procedures: Succinylcholine (succinylcholine chloride) Injection, 20 mg in each mL.
- Multiple-dose vials of 10 mL, box of 10 vials (NDC 0781-3009-95).
Storage
- Store in refrigerator at 2° to 8°C (36° to 46°F). The multi-dose vials are stable for up to 14 days at room temperature without significant loss of potency.
- Manufactured by
- Agila Specialties Pvt. Ltd.
- (Specialty Formulation Facility)
- Bangalore – 560 105, India for
- Sandoz Inc.
- Princeton, NJ 08540
- 04-2011
- 1016206
- Manufactured by
- Agila Specialties Pvt. Ltd.
- (Sterile Product Division)
- Bangalore – 560 076, India for
- Sandoz Inc.
- Princeton, NJ 08540
- 04-2011
- 1016227
- 200 mg Label
Images
Drug Images
{{#ask: Page Name::Suxamethonium chloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Suxamethonium chloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Suxamethonium chloride Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Suxamethonium chloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Suxamethonium chloride Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Suxamethonium chloride Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Suxamethonium chloride
|Pill Name=
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Suxamethonium chloride |Label Name=Succinyl choline label.png
}}