Sulindac: Difference between revisions
No edit summary |
No edit summary |
||
| Line 411: | Line 411: | ||
|structure= | |structure= | ||
* Sulindac is a non-steroidal, anti-inflammatory indene derivative designated chemically as (Z)-5-fluoro-2-methyl-1- | * Sulindac is a non-steroidal, anti-inflammatory indene derivative designated chemically as (Z)-5-fluoro-2-methyl-1-[ρ-(methylsulfinyl)phenyl]methylene]-1H-indene-3-acetic acid. It is not a salicylate, pyrazolone or propionic acid derivative. Its empirical formula is C20H17FO3S, with a molecular weight of 356.42. Sulindac, a yellow crystalline compound, is a weak organic acid practically insoluble in water below pH 4.5, but very soluble as the sodium salt or in buffers of pH 6 or higher. | ||
*Sulindac is available in 150 and 200 mg tablets for oral administration. Each tablet contains the following inactive ingredients: magnesium stearate, microcrystalline cellulose, plasdone and sodium starch glycolate. | *Sulindac is available in 150 and 200 mg tablets for oral administration. Each tablet contains the following inactive ingredients: magnesium stearate, microcrystalline cellulose, plasdone and sodium starch glycolate. | ||
Revision as of 15:24, 26 November 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
|
Overview
Sulindac is a non-steroidal, anti-inflammatory drug that is FDA approved for the {{{indicationType}}} of . There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition1
- Dosing Information
- Dosage
Condition2
- Dosing Information
- Dosage
Condition3
- Dosing Information
- Dosage
Condition4
- Dosing Information
- Dosage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Sulindac in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sulindac in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Sulindac in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Sulindac in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sulindac in pediatric patients.
Contraindications
- Condition1
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
|
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Sulindac in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Sulindac in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sulindac in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sulindac during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Sulindac with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Sulindac with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Sulindac with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Sulindac with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sulindac with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sulindac in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sulindac in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sulindac in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sulindac in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Sulindac in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Sulindac in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Sulindac in the drug label.
Pharmacology
There is limited information regarding Sulindac Pharmacology in the drug label.
Mechanism of Action
- Sulindac is a non-steroidal anti-inflammatory drug (NSAID) that exhibits anti-inflammatory, analgesic and antipyretic activities in animal models. The mechanism of action, like that of other NSAIDs, is not completely understood but may be related to prostaglandin synthetase inhibition.
Structure
- Sulindac is a non-steroidal, anti-inflammatory indene derivative designated chemically as (Z)-5-fluoro-2-methyl-1-[ρ-(methylsulfinyl)phenyl]methylene]-1H-indene-3-acetic acid. It is not a salicylate, pyrazolone or propionic acid derivative. Its empirical formula is C20H17FO3S, with a molecular weight of 356.42. Sulindac, a yellow crystalline compound, is a weak organic acid practically insoluble in water below pH 4.5, but very soluble as the sodium salt or in buffers of pH 6 or higher.
- Sulindac is available in 150 and 200 mg tablets for oral administration. Each tablet contains the following inactive ingredients: magnesium stearate, microcrystalline cellulose, plasdone and sodium starch glycolate.
- Following absorption, sulindac undergoes two major biotransformations - reversible reduction to the sulfide metabolite, and irreversible oxidation to the sulfone metabolite. Available evidence indicates that the biological activity resides with the sulfide metabolite.
- The structural formulas of sulindac and its metabolites are:
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Sulindac in the drug label.
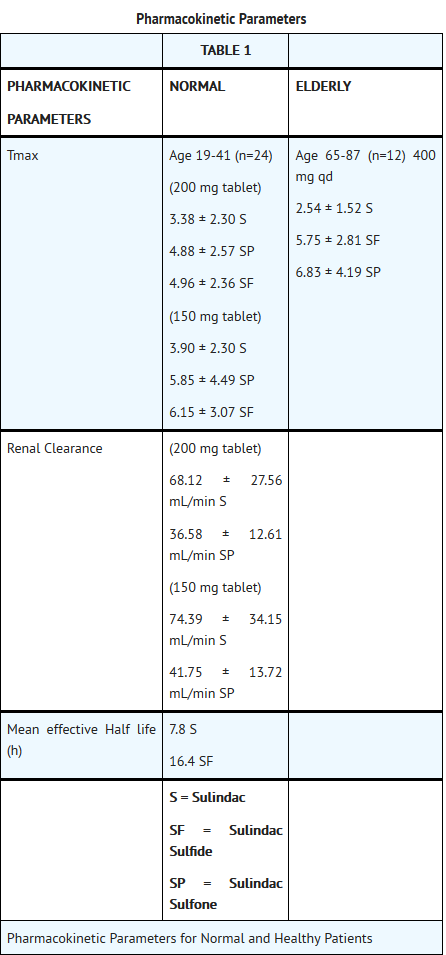
Pharmacokinetics
- Absorption
- The extent of sulindac absorption from sulindac tablets, USP is similar as compared to sulindac solution.
- There is no information regarding food effect on sulindac absorption. Antacids containing magnesium hydroxide 200 mg and aluminum hydroxide 225 mg per 5 mL have been shown not to significantly decrease the extent of sulindac absorption.
T1
Distribution
Sulindac, and its sulfone and sulfide metabolites, are 93.1, 95.4, and 97.9% bound to plasma proteins, predominantly to albumin. Plasma protein binding measured over a concentration range (0.5-2.0 µg/mL) was constant. Following an oral, radiolabeled dose of sulindac in rats, concentrations of radiolabel in red blood cells were about 10% of those in plasma. Sulindac penetrates the blood-brain and placental barriers. Concentrations in brain did not exceed 4% of those in plasma. Plasma concentrations in the placenta and in the fetus were less than 25% and 5% respectively, of systemic plasma concentrations. Sulindac is excreted in rat milk; concentrations in milk were 10 to 20% of those levels in plasma. It is not known if sulindac is excreted in human milk.
Metabolism
Sulindac undergoes two major biotransformations of its sulfoxide moiety: oxidation to the inactive sulfone and reduction to the pharmacologically active sulfide. The latter is readily reversible in animals and in man. These metabolites are present as unchanged compounds in plasma and principally as glucuronide conjugates in human urine and bile. A dihydroxydihydro analog has also been identified as a minor metabolite in human urine.
With the twice-a-day dosage regimen, plasma concentrations of sulindac and its two metabolites accumulate: mean concentration over a dosage interval at steady state relative to the first dose averages 1.5 and 2.5 times higher, respectively, for sulindac and its active sulfide metabolite.
Sulindac and its sulfone metabolite undergo extensive enterohepatic circulation relative to the sulfide metabolite in animals. Studies in man have also demonstrated that recirculation of the parent drug sulindac and its sulfone metabolite is more extensive than that of the active sulfide metabolite. The active sulfide metabolite accounts for less than six percent of the total intestinal exposure to sulindac and its metabolites.
Biochemical as well as pharmacological evidence indicates that the activity of sulindac resides in its sulfide metabolite. An in-vitro assay for inhibition of cyclooxygenase activity exhibited an EC50 of 0.02µM for sulindac sulfide. In-vivo models of inflammation indicate that activity is more highly correlated with concentrations of the metabolite than with parent drug concentrations.
Elimination
Approximately 50% of the administered dose of sulindac is excreted in the urine with the conjugated sulfone metabolite accounting for the major portion. Less than 1% of the administered dose of sulindac appears in the urine as the sulfide metabolite. Approximately 25% is found in the feces, primarily as the sulfone and sulfide metabolites.
The mean effective half life (T1/2) is 7.8 and 16.4 hours, respectively, for sulindac and its active sulfide metabolite.
Because sulindac is excreted in the urine primarily as biologically inactive forms, it may possibly affect renal function to a lesser extent than other non-steroidal anti-inflammatory drugs; however, renal adverse experiences have been reported with sulindac (see ADVERSE REACTIONS).
In a study of patients with chronic glomerular disease treated with therapeutic doses of sulindac, no effect was demonstrated on renal blood flow, glomerular filtration rate, or urinary excretion of prostaglandin E2 and the primary metabolite of prostacyclin, 6-keto-PGF1α. However, in other studies in healthy volunteers and patients with liver disease, sulindac was found to blunt the renal responses to intravenous furosemide, i.e., the diuresis, natriuresis, increments in plasma renin activity and urinary excretion of prostaglandins. These observations may represent a differentiation of the effects of sulindac on renal functions based on differences in pathogenesis of the renal prostaglandin dependence associated with differing dose-response relationships of different NSAIDs to the various renal functions influenced by prostaglandins (see PRECAUTIONS).
In healthy men, the average fecal blood loss, measured over a two-week period during administration of 400 mg per day of sulindac, was similar to that for placebo, and was statistically significantly less than that resulting from 4800 mg per day of aspirin.
Special Populations
Pediatrics
The pharmacokinetics of sulindac have not been investigated in pediatric patients.
Race
Pharmacokinetic differences due to race have not been identified.
Hepatic Insufficiency
Patients with acute and chronic hepatic disease may require reduced doses of sulindac compared to patients with normal hepatic function since hepatic metabolism is an important elimination pathway.
Following a single dose, plasma concentrations of the active sulfide metabolite have been reported to be higher in patients with alcoholic liver disease compared to healthy normal subjects.
Renal Insufficiency
Sulindac pharmacokinetics have been investigated in patients with renal insufficiency. The disposition of sulindac was studied in end-stage renal disease patients requiring hemodialysis. Plasma concentrations of sulindac and its sulfone metabolite were comparable to those of normal healthy volunteers whereas concentrations of the active sulfide metabolite were significantly reduced. Plasma protein binding was reduced and the AUC of the unbound sulfide metabolite was about half that in healthy subjects.
Sulindac and its metabolites are not significantly removed from the blood in patients undergoing hemodialysis.
Since sulindac is eliminated primarily by the kidneys, patients with significantly impaired renal function should be closely monitored.
A lower daily dosage should be anticipated to avoid excessive drug accumulation.
In controlled clinical studies sulindac was evaluated in the following five conditions:
1. Osteoarthritis
In patients with osteoarthritis of the hip and knee, the anti-inflammatory and analgesic activity of sulindac was demonstrated by clinical measurements that included: assessments by both patient and investigator of overall response; decrease in disease activity as assessed by both patient and investigator; improvement in ARA Functional Class; relief of night pain; improvement in overall evaluation of pain, including pain on weight bearing and pain on active and passive motion; improvement in joint mobility, range of motion, and functional activities; decreased swelling and tenderness; and decreased duration of stiffness following prolonged inactivity.
In clinical studies in which dosages were adjusted according to patient needs, sulindac, 200 to 400 mg daily was shown to be comparable in effectiveness to aspirin 2400 to 4800 mg daily. Sulindac was generally well tolerated, and patients on it had a lower overall incidence of total adverse effects, of milder gastrointestinal reactions, and of tinnitus than did patients on aspirin. (See ADVERSE REACTIONS)
2. Rheumatoid Arthritis
In patients with rheumatoid arthritis, the anti-inflammatory and analgesic activity of sulindac was demonstrated by clinical measurements that included: assessments by both patient and investigator of overall response; decrease in disease activity as assessed by both patient and investigator; reduction in overall joint pain; reduction in duration and severity of morning stiffness; reduction in day and night pain; decrease in time required to walk 50 feet; decrease in general pain as measured on a visual analog scale; improvement in the Ritchie articular index; decrease in proximal interphalangeal joint size; improvement in ARA Functional Class; increase in grip strength; reduction in painful joint count and score; reduction in swollen joint count and score; and increased flexion and extension of the wrist.
In clinical studies in which dosages were adjusted according to patient needs, sulindac 300 to 400 mg daily was shown to be comparable in effectiveness to aspirin 3600 to 4800 mg daily. Sulindac was generally well tolerated, and patients on it had a lower overall incidence of total adverse effects, of milder gastrointestinal reactions, and of tinnitus than did patients on aspirin. (See ADVERSE REACTIONS)
In patients with rheumatoid arthritis, sulindac may be used in combination with gold salts at usual dosage levels. In clinical studies, sulindac added to the regimen of gold salts usually resulted in additional symptomatic relief but did not alter the course of the underlying disease.
3. Ankylosing Spondylitis
In patients with ankylosing spondylitis, the anti-inflammatory and analgesic activity of sulindac was demonstrated by clinical measurements that included: assessments by both patient and investigator of overall response; decrease in disease activity as assessed by both patient and investigator; improvement in ARA Functional Class; improvement in patient and investigator evaluation of spinal pain, tenderness and/or spasm; reduction in the duration of morning stiffness; increase in the time to onset of fatigue; relief of night pain; increase in chest expansion; and increase in spinal mobility evaluated by fingers-to-floor distance, occiput to wall distance, the Schober Test, and the Wright Modification of the Schober Test. In a clinical study in which dosages were adjusted according to patient need, sulindac 200 to 400 mg daily was as effective as indomethacin 75 to 150 mg daily. In a second study, sulindac 300 to 400 mg daily was comparable in effectiveness to phenylbutazone 400 to 600 mg daily. Sulindac was better tolerated than phenylbutazone. (See ADVERSE REACTIONS)
4. Acute Painful Shoulder (Acute subacromial bursitis/supraspinatus tendinitis
In patients with acute painful shoulder (acute subacromial bursitis/supraspinatus tendinitis), the anti-inflammatory and analgesic activity of sulindac was demonstrated by clinical measurements that included: assessments by both patient and investigator of overall response; relief of night pain, spontaneous pain, and pain on active motion; decrease in local tenderness; and improvement in range of motion measured by abduction, and internal and external rotation. In clinical studies in acute painful shoulder, sulindac 300 to 400 mg daily and oxyphenbutazone 400 to 600 mg daily were shown to be equally effective and well tolerated.
5. Acute gouty arthritis
In patients with acute gouty arthritis, the anti-inflammatory and analgesic activity of sulindac was demonstrated by clinical measurements that included: assessments by both the patient and investigator of overall response; relief of weight-bearing pain; relief of pain at rest and on active and passive motion; decrease in tenderness; reduction in warmth and swelling; increase in range of motion; and improvement in ability to function. In clinical studies, sulindac at 400 mg daily and phenylbutazone at 600 mg daily were shown to be equally effective. In these short-term studies in which reduction of dosage was permitted according to response, both drugs were equally well tolerated.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Sulindac in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Sulindac in the drug label.
How Supplied
Storage
There is limited information regarding Sulindac Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Sulindac |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sulindac |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Sulindac in the drug label.
Precautions with Alcohol
- Alcohol-Sulindac interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Sulindac |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Sulindac |Label Name=Sulindac11.png
}}
{{#subobject:
|Label Page=Sulindac |Label Name=Sulindac11.png
}}