Sufentanil: Difference between revisions
No edit summary |
No edit summary |
||
| Line 25: | Line 25: | ||
* Intravenous administration or unintentional intravascular injection during epidural administration of Sufentanil may cause skeletal muscle rigidity, particularly of the truncal muscles. The incidence and severity of muscle rigidity is dose related. Administration of Sufentanil may produce muscular rigidity with a more rapid onset of action than that seen with [[fentanyl]]. Sufentanil may produce muscular rigidity that involves the skeletal muscles of the neck and extremities. As with fentanyl, muscular rigidity has been reported to occur or recur infrequently in the extended postoperative period. The incidence of muscular rigidity associated with intravenous Sufentanil can be reduced by: 1) administration of up to 1/4 of the full paralyzing dose of a non-depolarizing neuromuscular blocking agent just prior to administration of Sufentanil at dosages of up to 8 mcg/kg, 2) administration of a full paralyzing dose of a neuromuscular blocking agent following loss of consciousness when Sufentanil is used in anesthetic dosages (above 8 mcg/kg) titrated by slow intravenous infusion, or, 3) simultaneous administration of Sufentanil and a full paralyzing dose of a neuromuscular blocking agent when Sufentanil is used in rapidly administered anesthetic dosages (above 8 mcg/kg). | * Intravenous administration or unintentional intravascular injection during epidural administration of Sufentanil may cause skeletal muscle rigidity, particularly of the truncal muscles. The incidence and severity of muscle rigidity is dose related. Administration of Sufentanil may produce muscular rigidity with a more rapid onset of action than that seen with [[fentanyl]]. Sufentanil may produce muscular rigidity that involves the skeletal muscles of the neck and extremities. As with fentanyl, muscular rigidity has been reported to occur or recur infrequently in the extended postoperative period. The incidence of muscular rigidity associated with intravenous Sufentanil can be reduced by: 1) administration of up to 1/4 of the full paralyzing dose of a non-depolarizing neuromuscular blocking agent just prior to administration of Sufentanil at dosages of up to 8 mcg/kg, 2) administration of a full paralyzing dose of a neuromuscular blocking agent following loss of consciousness when Sufentanil is used in anesthetic dosages (above 8 mcg/kg) titrated by slow intravenous infusion, or, 3) simultaneous administration of Sufentanil and a full paralyzing dose of a neuromuscular blocking agent when Sufentanil is used in rapidly administered anesthetic dosages (above 8 mcg/kg). | ||
* The neuromuscular blocking agents used should be compatible with the patient's cardiovascular status. Adequate facilities should be available for postoperative monitoring and ventilation of patients administered Sufentanil. It is essential that these facilities be fully equipped to handle all degrees of respiratory depression. | * The neuromuscular blocking agents used should be compatible with the patient's cardiovascular status. Adequate facilities should be available for postoperative monitoring and ventilation of patients administered Sufentanil. It is essential that these facilities be fully equipped to handle all degrees of respiratory depression. | ||
|clinicalTrials=The most common adverse reactions of opioids are respiratory depression and skeletal muscle rigidity, particularly of the truncal muscles. | |clinicalTrials=* The most common adverse reactions of opioids are respiratory depression and skeletal muscle rigidity, particularly of the truncal muscles. Sufentanil may produce muscular rigidity that involves the skeletal muscles of the neck and extremities. See Clinical Pharmacology, Warnings And Precautions on the management of respiratory depression and skeletal muscle rigidity. Urinary retention has been associated with the use of epidural opioids but was not reported in the clinical trials of epidurally administered Sufentanill due to the use of indwelling catheters. The incidence of urinary retention in patients without urinary catheters receiving epidural Sufentanil is unknown; return of normal bladder activity may be delayed. | ||
* The following adverse reaction information is derived from controlled clinical trials in 320 patients who received intravenous Sufentanil during surgical anesthesia and in 340 patients who received epidural Sufentanil plus bupivacaine 0.125% for analgesia during labor and is presented below. Based on the observed frequency, none of the reactions occurring with an incidence less than 1% were observed during clinical trials of epidural Sufentanil used during labor and delivery (N=340). | |||
* In general cardiovascular and musculoskeletal adverse experiences were not observed in clinical trials of epidural Sufentanilnil. Hypotension was observed 7 times more frequently in intravenous trials than in epidural trials. The incidence of central nervous system, dermatological and gastrointestinal adverse experiences was approximately 4 to 25 times higher in studies of epidural use in labor and delivery. | |||
* Probably Causally Related: Incidence Greater than 1% - Derived from clinical trials (See preceding paragraph) | |||
* [[Cardiovascular]]: [[bradycardia]], [[hypertension]], [[hypotension]]. | |||
* [[Musculoskeletal]]: chest wall rigidity. | |||
* [[Central Nervous System]]: [[somnolence]]. | |||
* [[Dermatological]]: [[pruritus]] (25%). | |||
* [[Gastrointestinal]]: [[nausea]], [[vomiting]]. | |||
Central Nervous System: | |||
Dermatological: pruritus (25%). | |||
Gastrointestinal: | |||
Probably Causally Related: Incidence Less than 1% - Derived from clinical trials (Adverse events reported in post-marketing surveillance, not seen in clinical trials, are italicized.) | Probably Causally Related: Incidence Less than 1% - Derived from clinical trials (Adverse events reported in post-marketing surveillance, not seen in clinical trials, are italicized.) | ||
Revision as of 03:10, 11 June 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Sufentanil is a analgesic opioid that is FDA approved for the {{{indicationType}}} of analgesia in labor, epidural; adjunct, general anesthesia. Common adverse reactions include cardiovascular: bradyarrhythmia (3% to 9% ), hypotension (3% to 9% ), dermatologic: pruritus (25% ), gastrointestinal: nausea (3% to 9% ), vomiting (3% to 9% ), musculoskeletal: muscle rigidity, chest wall (3% to 9% ), neurologic: somnolence (3% to 9% ).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Dosage should be individualized
- Analgesia in labor, epidural; adjunct: (epidural use) 10-15 mcg sufentanil administered with 10 mL bupivacaine 0.125% with or without epinephrine; may repeat twice at not less than 1 hr intervals until delivery; max 3 doses
- General anesthesia: primary anesthetic agent, 8-30 mcg/kg IV with 100% oxygen and a muscle relaxant, then 0.5-10 mcg/kg as needed in response to signs of lightening of anesthesia; max 30 mcg/kg/procedure
- General anesthesia: analgesic adjunct to balanced general anesthesia, 1-8 mcg/kg IV (approximately 1 mcg/kg/hr of estimated surgical duration); 75% given prior to intubation, then incrementally as 10-50 mcg IV as needed in response to signs of lightening of analgesia
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Sufentanil in adult patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Sufentanil in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- General anesthesia: cardiovascular surgery (age under 12 yr) 10-25 mcg/kg with 100% oxygen, additional doses up to 25-50 mcg for maintenance of anesthesia
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Sufentanil in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Sufentanil in pediatric patients.
Contraindications
- Sufentanil is contraindicated in patients with known hypersensitivity to the drug or known intolerance to other opioid agonists.
Warnings
- Sufentanil should be administered only by persons specifically trained in the use of intravenous and epidural anesthetics and management of the respiratory effects of potent opioids.
- An opioid antagonist, resuscitative and intubation equipment and oxygen should be readily available.
- Prior to catheter insertion, the physician should be familiar with patient conditions (such as infection at the injection site, bleeding diathesis, anticoagulant therapy, etc.) which call for special evaluation of the benefit versus risk potential.
Intravenous use
- Intravenous administration or unintentional intravascular injection during epidural administration of Sufentanil may cause skeletal muscle rigidity, particularly of the truncal muscles. The incidence and severity of muscle rigidity is dose related. Administration of Sufentanil may produce muscular rigidity with a more rapid onset of action than that seen with fentanyl. Sufentanil may produce muscular rigidity that involves the skeletal muscles of the neck and extremities. As with fentanyl, muscular rigidity has been reported to occur or recur infrequently in the extended postoperative period. The incidence of muscular rigidity associated with intravenous Sufentanil can be reduced by: 1) administration of up to 1/4 of the full paralyzing dose of a non-depolarizing neuromuscular blocking agent just prior to administration of Sufentanil at dosages of up to 8 mcg/kg, 2) administration of a full paralyzing dose of a neuromuscular blocking agent following loss of consciousness when Sufentanil is used in anesthetic dosages (above 8 mcg/kg) titrated by slow intravenous infusion, or, 3) simultaneous administration of Sufentanil and a full paralyzing dose of a neuromuscular blocking agent when Sufentanil is used in rapidly administered anesthetic dosages (above 8 mcg/kg).
- The neuromuscular blocking agents used should be compatible with the patient's cardiovascular status. Adequate facilities should be available for postoperative monitoring and ventilation of patients administered Sufentanil. It is essential that these facilities be fully equipped to handle all degrees of respiratory depression.
Adverse Reactions
Clinical Trials Experience
- The most common adverse reactions of opioids are respiratory depression and skeletal muscle rigidity, particularly of the truncal muscles. Sufentanil may produce muscular rigidity that involves the skeletal muscles of the neck and extremities. See Clinical Pharmacology, Warnings And Precautions on the management of respiratory depression and skeletal muscle rigidity. Urinary retention has been associated with the use of epidural opioids but was not reported in the clinical trials of epidurally administered Sufentanill due to the use of indwelling catheters. The incidence of urinary retention in patients without urinary catheters receiving epidural Sufentanil is unknown; return of normal bladder activity may be delayed.
- The following adverse reaction information is derived from controlled clinical trials in 320 patients who received intravenous Sufentanil during surgical anesthesia and in 340 patients who received epidural Sufentanil plus bupivacaine 0.125% for analgesia during labor and is presented below. Based on the observed frequency, none of the reactions occurring with an incidence less than 1% were observed during clinical trials of epidural Sufentanil used during labor and delivery (N=340).
- In general cardiovascular and musculoskeletal adverse experiences were not observed in clinical trials of epidural Sufentanilnil. Hypotension was observed 7 times more frequently in intravenous trials than in epidural trials. The incidence of central nervous system, dermatological and gastrointestinal adverse experiences was approximately 4 to 25 times higher in studies of epidural use in labor and delivery.
- Probably Causally Related: Incidence Greater than 1% - Derived from clinical trials (See preceding paragraph)
- Cardiovascular: bradycardia, hypertension, hypotension.
- Musculoskeletal: chest wall rigidity.
- Central Nervous System: somnolence.
- Dermatological: pruritus (25%).
- Gastrointestinal: nausea, vomiting.
Probably Causally Related: Incidence Less than 1% - Derived from clinical trials (Adverse events reported in post-marketing surveillance, not seen in clinical trials, are italicized.)
Body as a whole: anaphylaxis.
Cardiovascular: arrhythmia2, tachycardia2, cardiac arrest.
Central Nervous System: chills2.
Dermatological: erythema2.
Musculoskeletal: skeletal muscle rigidity of neck and extremities.
Respiratory: apnea2, bronchospasm2, postoperative respiratory depression2.
Miscellaneous: intraoperative muscle movement2.
Postmarketing Experience
There is limited information regarding Sufentanil Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Sufentanil Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C SUFENTA has been shown to have an embryocidal effect in rats and rabbits when given in doses 2.5 times the upper human intravenous dose for a period of 10 days to over 30 days. These effects were most probably due to maternal toxicity (decreased food consumption with increased mortality) following prolonged administration of the drug.
No evidence of teratogenic effects have been observed after administration of SUFENTA in rats or rabbits.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sufentanil in women who are pregnant.
Labor and Delivery
The use of epidurally administered SUFENTA in combination with bupivacaine 0.125% with or without epinephrine is indicated for labor and delivery. (See INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION sections.) SUFENTA is not recommended for intravenous use or for use of larger epidural doses during labor and delivery because of potential risks to the newborn infant after delivery. In clinical trials, one case of severe fetal bradycardia associated with maternal hypotension was reported within 8 minutes of maternal administration of sufentanil 15 mcg plus bupivacaine 0.125% (10 mL total volume).
Nursing Mothers
It is not known whether sufentanil is excreted in human milk. Because fentanyl analogs are excreted in human milk, caution should be exercised when SUFENTA is administered to a nursing woman.
Pediatric Use
The safety and efficacy of intravenous SUFENTA in pediatric patients as young as 1 day old undergoing cardiovascular surgery have been documented in a limited number of cases. The clearance of SUFENTA in healthy neonates is approximately one-half that in adults and children. The clearance rate of SUFENTA can be further reduced by up to a third in neonates with cardiovascular disease, resulting in an increase in the elimination half-life of the drug.
Geriatic Use
There is no FDA guidance on the use of Sufentanil in geriatric settings.
Gender
There is no FDA guidance on the use of Sufentanil with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sufentanil with respect to specific racial populations.
Renal Impairment
In patients with liver or kidney dysfunction, SUFENTA should be administered with caution due to the importance of these organs in the metabolism and excretion of SUFENTA
Hepatic Impairment
In patients with liver or kidney dysfunction, SUFENTA should be administered with caution due to the importance of these organs in the metabolism and excretion of SUFENTA
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sufentanil in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sufentanil in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Sufentanil Administration in the drug label.
Monitoring
There is limited information regarding Sufentanil Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Sufentanil and IV administrations.
Overdosage
Overdosage is manifested by an extension of the pharmacological actions of SUFENTA (see CLINICAL PHARMACOLOGY) as with other potent opioid analgesics. The most serious and significant effect of overdose for both intravenous and epidural administration of SUFENTA is respiratory depression. Intravenous administration of an opioid antagonist such as naloxone should be employed as a specific antidote to manage respiratory depression. The duration of respiratory depression following overdosage with SUFENTA may be longer than the duration of action of the opioid antagonist. Administration of an opioid antagonist should not preclude more immediate countermeasures. In the event of overdosage, oxygen should be administered and ventilation assisted or controlled as indicated for hypoventilation or apnea. A patent airway must be maintained, and a nasopharyngeal airway or endotracheal tube may be indicated. If depressed respiration is associated with muscular rigidity, a neuromuscular blocking agent may be required to facilitate assisted or controlled respiration. Intravenous fluids and vasopressors for the treatment of hypotension and other supportive measures may be employed.
Pharmacology
| |
| |
Sufentanil
| |
| Systematic (IUPAC) name | |
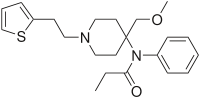
| N-[4-(Methoxymethyl)-1-(2-thiofuran-2-ylethyl)-4-piperidyl]-N-phenylpropanamide | |
| Identifiers | |
| CAS number | |
| ATC code | N01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 386.552 g/mol |
| SMILES | & |
| Physical data | |
| Melt. point | 97 °C (207 °F) |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 162 minutes |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(US) |
| Legal status | |
| Routes | IV, IM, SubQ, epidural, intrathecal, transdermal patch (in clinical trials) |
Mechanism of Action
SUFENTA is an opioid analgesic. When used in balanced general anesthesia, SUFENTA has been reported to be as much as 10 times as potent as fentanyl. When administered intravenously as a primary anesthetic agent with 100% oxygen, SUFENTA is approximately 5 to 7 times as potent as fentanyl.
Assays of histamine in patients administered SUFENTA have shown no elevation in plasma histamine levels and no indication of histamine release.
(See dosage chart for more complete information on the intravenous use of SUFENTA.)
Structure
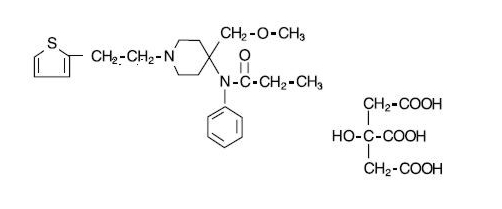
SUFENTA® (sufentanil citrate) is a potent opioid analgesic chemically designated as N-[4-(methyoxymethyl)-1-[2-(2-thienyl)ethyl]-4-piperidinyl]-N-phenylpropanamide:2-hydroxy-1,2,3-propanetricarboxylate (1:1) with a molecular weight of 578.68. The structural formula of SUFENTA is:
SUFENTA is a sterile, preservative free, aqueous solution containing sufentanil citrate equivalent to 50 mcg per mL of sufentanil base for intravenous and epidural injection. The solution has a pH range of 3.5 to 6.0.
Pharmacodynamics
Intravenous use
At intravenous doses of up to 8 mcg/kg, SUFENTA is an analgesic component of general anesthesia; at intravenous doses ≥8 mcg/kg, SUFENTA produces a deep level of anesthesia. SUFENTA produces a dose related attenuation of catecholamine release, particularly norepinephrine.
At intravenous dosages of ≥8 mcg/kg, SUFENTA produces hypnosis and anesthesia without the use of additional anesthetic agents. A deep level of anesthesia is maintained at these dosages, as demonstrated by EEG patterns. Dosages of up to 25 mcg/kg attenuate the sympathetic response to surgical stress. The catecholamine response, particularly norepinephrine, is further attenuated at doses of SUFENTA of 25 to 30 mcg/kg, with hemodynamic stability and preservation of favorable myocardial oxygen balance.
SUFENTA has an immediate onset of action, with relatively limited accumulation. Rapid elimination from tissue storage sites allows for relatively more rapid recovery as compared with equipotent dosages of fentanyl. At dosages of 1 to 2 mcg/kg, recovery times are comparable to those observed with fentanyl; at dosages of >2 to 6 mcg/kg, recovery times are comparable to enflurane, isoflurane and fentanyl. Within the anesthetic dosage range of 8 to 30 mcg/kg of SUFENTA, recovery times are more rapid compared to equipotent fentanyl dosages.
The vagolytic effects of pancuronium may produce a dose dependent elevation in heart rate during SUFENTA-oxygen anesthesia. The use of moderate doses of pancuronium or of a less vagolytic neuromuscular blocking agent may be used to maintain a stable lower heart rate and blood pressure during SUFENTA-oxygen anesthesia. The vagolytic effects of pancuronium may be reduced in patients administered nitrous oxide with SUFENTA.
Preliminary data suggest that in patients administered high doses of SUFENTA, initial dosage requirements for neuromuscular blocking agents are generally lower as compared to patients given fentanyl or halothane, and comparable to patients given enflurane.
Bradycardia is infrequently seen in patients administered SUFENTA-oxygen anesthesia. The use of nitrous oxide with high doses of SUFENTA may decrease mean arterial pressure, heart rate and cardiac output.
SUFENTA at 20 mcg/kg has been shown to provide more adequate reduction in intracranial volume than equivalent doses of fentanyl, based upon requirements for furosemide and anesthesia supplementation in one study of patients undergoing craniotomy. During carotid endarterectomy, SUFENTA-nitrous oxide/oxygen produced reductions in cerebral blood flow comparable to those of enflurane-nitrous oxide/oxygen. During cardiovascular surgery, SUFENTA-oxygen produced EEG patterns similar to fentanyl-oxygen; these EEG changes were judged to be compatible with adequate general anesthesia.
The intraoperative use of SUFENTA at anesthetic dosages maintains cardiac output, with a slight reduction in systemic vascular resistance during the initial postoperative period. The incidence of postoperative hypertension, need for vasoactive agents and requirements for postoperative analgesics are generally reduced in patients administered moderate or high doses of SUFENTA as compared to patients given inhalation agents.
Skeletal muscle rigidity is related to the dose and speed of administration of SUFENTA. This muscular rigidity may occur unless preventative measures are taken (see WARNINGS).
Decreased respiratory drive and increased airway resistance occur with SUFENTA. The duration and degree of respiratory depression are dose related when SUFENTA is used at sub-anesthetic dosages. At high doses, a pronounced decrease in pulmonary exchange and apnea may be produced.
Epidural use in Labor and Delivery
Onset of analgesic effect occurs within approximately 10 minutes of administration of epidural doses of SUFENTA and bupivacaine. Duration of analgesia following a single epidural injection of 10 to 15 mcg SUFENTA and bupivacaine 0.125% averaged 1.7 hours.
During labor and vaginal delivery, the addition of 10 to 15 mcg SUFENTA to 10 mL 0.125% bupivacaine provides an increase in the duration of analgesia compared to bupivacaine without an opioid. Analgesia from 15 mcg SUFENTA plus 10 mL 0.125% bupivacaine is comparable to analgesia from 10 mL of 0.25% bupivacaine alone. Apgar scores of neonates following epidural administration of both drugs to women in labor were comparable to neonates whose mothers received bupivacaine without an opioid epidurally.
Pharmacokinetics
Intravenous use
The pharmacokinetics of intravenous SUFENTA can be described as a three-compartment model, with a distribution time of 1.4 minutes, redistribution of 17.1 minutes and elimination half-life of 164 minutes in adults. The elimination half-life of SUFENTA is shorter (e.g. 97 +/- 42 minutes) in infants and children, and longer in neonates (e.g. 434 +/- 160 minutes) compared to that of adolescents and adults. The liver and small intestine are the major sites of biotransformation. Approximately 80% of the administered dose is excreted within 24 hours and only 2% of the dose is eliminated as unchanged drug. Plasma protein binding of sufentanil, related to the alpha acid glycoprotein concentration, was approximately 93% in healthy males, 91% in mothers and 79% in neonates.
Epidural use in Labor and Delivery
After epidural administration of incremental doses totaling 5 to 40 mcg SUFENTA during labor and delivery, maternal and neonatal sufentanil plasma concentrations were at or near the 0.05 to 0.1 ng/mL limit of detection, and were slightly higher in mothers than in their infants.
Nonclinical Toxicology
There is limited information regarding Sufentanil Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Sufentanil Clinical Studies in the drug label.
How Supplied
SUFENTA (Sufentanil Citrate Injection, USP) is supplied as a sterile aqueous preservative-free solution for intravenous and epidural use as:
NDC 11098-050-01 50 mcg/mL sufentanil base, 1 mL ampules in packages of 10 NDC 11098-050-02 50 mcg/mL sufentanil base, 2 mL ampules in packages of 10 NDC 11098-050-05 50 mcg/mL sufentanil base, 5 mL ampules in packages of 10
Storage
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature]. PROTECT FROM LIGHT.
U.S. Patent No. 3,998,834
MAY 1995, SEPTEMBER 1995
TAYLOR PHARMACEUTICALS AN AKORN COMPANY Decatur, IL 62522
SFA0N
Rev. 07/07
Images
Drug Images
{{#ask: Page Name::Sufentanil |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sufentanil |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Sufentanil Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Sufentanil interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Sufentanil Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Sufentanil Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Sufentanil |Label Name=Sulfentanil label.png
}}