Sodium oxybate (patient information): Difference between revisions
m (Protected "Sodium oxybate (patient information)": Robot: Protecting all pages from category Drug ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
|authorTag={{AP}} | |||
|genericName={{AP}} | |||
|aOrAn=an | |||
|drugClass=[[antibacterial]] | |||
|indication=for hand washing to decrease bacteria on the skin | |||
|blackBoxWarningTitle=<b><span style="color:#FF0000;">TITLE</span></b> | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">Condition Name:</span></i> (Content) | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Sodium oxybate (patient information) in adult patients. | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Sodium oxybate (patient information) in adult patients. | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of Sodium oxybate (patient information) in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of Sodium oxybate (patient information) in pediatric patients. | |||
|warnings=*For external use only. | |||
*When using this product: | |||
**Avoid contact with eyes. | |||
**In case of eye contact, flush eyes with water. | |||
*Stop use and ask a doctor if irritation or redness develops, or if condition persists for more than 72 hours. | |||
*Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. | |||
|drugBox={{Chembox | |||
| ImageFile = Chloroxylenol.svg | |||
| ImageFile_Ref = {{chemboximage|correct|??}} | |||
| ImageSize = 100 | |||
| ImageName = Kekulé, skeletal formula of chloroxylenol | |||
| SystematicName = 4-Chloro-3,5-dimethylphenol<ref>{{Cite web|title = chloroxylenol – Compound Summary|url = http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=2723&loc=ec_rcs|work = PubChem Compound|publisher = National Center for Biotechnology Information|accessdate = 7 October 2011|location = USA|date = 25 March 2005|at = Identification and Related Records}}</ref> | |||
| Section1 = {{Chembox Identifiers | |||
| CASNo = 88-04-0 | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| PubChem = 2723 | |||
| PubChem_Ref = {{Pubchemcite|correct|Pubchem}} | |||
| ChemSpiderID = 21106017 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| UNII = 0F32U78V2Q | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| EINECS = 201-793-8 | |||
| KEGG = D03473 | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| MeSHName = chloroxylenol | |||
| ChEBI = 34393 | |||
| ChEMBL = 398440 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| RTECS = ZE6850000 | |||
| Beilstein = 1862539 | |||
| ATCCode_prefix = D08 | |||
| ATCCode_suffix = AE05 | |||
| SMILES = Cc1cc(O)cc(C)c1Cl | |||
| SMILES1 = CC1=CC(O)=CC(C)=C1Cl | |||
| StdInChI = 1S/C8H9ClO/c1-5-3-7(10)4-6(2)8(5)9/h3-4,10H,1-2H3 | |||
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| InChI = 1/C8H9ClO/c1-5-3-7(10)4-6(2)8(5)9/h3-4,10H,1-2H3 | |||
| StdInChIKey = OSDLLIBGSJNGJE-UHFFFAOYSA-N | |||
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| InChIKey = OSDLLIBGSJNGJE-UHFFFAOYAY | |||
}} | |||
| Section2 = {{Chembox Properties | |||
| C=8|H=9|Cl=1|O=1 | |||
| ExactMass = 156.034192617 g mol<sup>-1</sup> | |||
| MeltingPtC = 114 | |||
| MeltingPtCH = 116 | |||
| LogP = 3.377 | |||
| pKa = 9.76 | |||
| pKb = 4.24 | |||
}} | |||
| Section3 = {{Chembox Hazards | |||
| GHSPictograms = {{GHS exclamation mark}} | |||
| GHSSignalWord = '''WARNING''' | |||
| HPhrases = {{H-phrases|302|315|317|319}} | |||
| PPhrases = {{P-phrases|280|305+351+338}} | |||
| EUIndex = 604-038-00-4 | |||
| EUClass = {{Hazchem Xn}} | |||
| RPhrases = {{R22}}, {{R36/38}}, {{R43}} | |||
| SPhrases = {{S2}}, {{S24}}, {{S37}} | |||
}} | |||
}} | |||
|packLabel=[[file:Appearance Chloroxylenil.png|none|400px]] | |||
|alcohol=Alcohol-Sodium oxybate (patient information) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
}} | |||
{{drug header}} | {{drug header}} | ||
Revision as of 21:27, 28 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Sodium oxybate (patient information) is an antibacterial that is FDA approved for the {{{indicationType}}} of for hand washing to decrease bacteria on the skin. Common adverse reactions include {{{adverseReactions}}}.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Sodium oxybate (patient information) FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sodium oxybate (patient information) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sodium oxybate (patient information) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Sodium oxybate (patient information) FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sodium oxybate (patient information) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sodium oxybate (patient information) in pediatric patients.
Contraindications
There is limited information regarding Sodium oxybate (patient information) Contraindications in the drug label.
Warnings
- For external use only.
- When using this product:
- Avoid contact with eyes.
- In case of eye contact, flush eyes with water.
- Stop use and ask a doctor if irritation or redness develops, or if condition persists for more than 72 hours.
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Sodium oxybate (patient information) Clinical Trials Experience in the drug label.
Postmarketing Experience
There is limited information regarding Sodium oxybate (patient information) Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Sodium oxybate (patient information) Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Sodium oxybate (patient information) in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sodium oxybate (patient information) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sodium oxybate (patient information) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Sodium oxybate (patient information) in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Sodium oxybate (patient information) in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Sodium oxybate (patient information) in geriatric settings.
Gender
There is no FDA guidance on the use of Sodium oxybate (patient information) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sodium oxybate (patient information) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sodium oxybate (patient information) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sodium oxybate (patient information) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sodium oxybate (patient information) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sodium oxybate (patient information) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Sodium oxybate (patient information) Administration in the drug label.
Monitoring
There is limited information regarding Sodium oxybate (patient information) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Sodium oxybate (patient information) and IV administrations.
Overdosage
There is limited information regarding Sodium oxybate (patient information) overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Template:Chembox BeilsteinTemplate:Chembox ECNumberTemplate:Chembox E numberTemplate:Chembox RTECSTemplate:Chembox MeltingPtTemplate:Chembox LogPTemplate:Chembox pKaTemplate:Chembox pKbTemplate:Chembox GHSPictogramsTemplate:Chembox GHSSignalWordTemplate:Chembox HPhrasesTemplate:Chembox PPhrases| Kekulé, skeletal formula of chloroxylenol | |
| Names | |
|---|---|
| Systematic IUPAC name
4-Chloro-3,5-dimethylphenol[1] | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
| KEGG | |
| MeSH | chloroxylenol |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H9ClO | |
| Molar mass | 156.61 g·mol−1 |
| Hazards | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mechanism of Action
There is limited information regarding Sodium oxybate (patient information) Mechanism of Action in the drug label.
Structure
There is limited information regarding Sodium oxybate (patient information) Structure in the drug label.
Pharmacodynamics
There is limited information regarding Sodium oxybate (patient information) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Sodium oxybate (patient information) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Sodium oxybate (patient information) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Sodium oxybate (patient information) Clinical Studies in the drug label.
How Supplied
There is limited information regarding Sodium oxybate (patient information) How Supplied in the drug label.
Storage
There is limited information regarding Sodium oxybate (patient information) Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Sodium oxybate (patient information) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
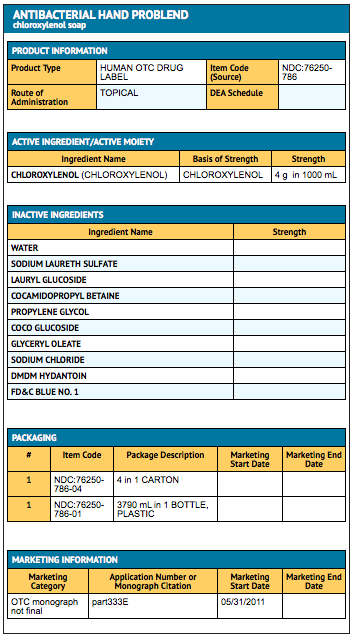
{{#ask: Label Page::Sodium oxybate (patient information) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Sodium oxybate (patient information) Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Sodium oxybate (patient information) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Sodium oxybate (patient information) Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Sodium oxybate (patient information) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "chloroxylenol – Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 25 March 2005. Identification and Related Records. Retrieved 7 October 2011.
IMPORTANT WARNING:
Sodium oxybate is another name for GHB, a substance that is often illegally sold and abused, especially by young adults in social settings such as nightclubs. Sodium oxybate may be harmful when taken by people other than the person for whom it was prescribed. Do not sell or give your sodium oxybate to anyone else; selling or sharing it is against the law. Store sodium oxybate in a safe place, such as a locked cabinet or box, so that no one else can take it accidentally or on purpose. Keep track of how much liquid is left in your bottle so you will know if any is missing.
Take sodium oxybate exactly as directed. Do not take more of it or take it more often than prescribed by your doctor. If you take too much sodium oxybate, you may experience life-threatening symptoms including seizures, slowed or stopped breathing, loss of consciousness, and coma. You may also develop a craving for sodium oxybate, feel a need to take larger and larger doses, or want to continue taking sodium oxybate even though it causes unpleasant symptoms. If you have taken sodium oxybate in amounts larger than prescribed by your doctor, and you suddenly stop taking it, you may experience withdrawal symptoms such as difficulty falling asleep or staying asleep, restlessness, anxiety, abnormal thinking, loss of contact with reality, sleepiness, upset stomach, shaking of a part of your body that you cannot control, sweating, muscle cramps, and fast heartbeat.
Sodium oxybate may cause serious side effects even if it is taken as directed. Do not take antidepressants; medications for anxiety, mental illness, or seizures; sedatives; sleeping pills; or tranquilizers while you are taking sodium oxybate. Do not drink alcohol while you are taking sodium oxybate. Tell your doctor if you snore and if you have or have ever had lung disease, difficulty breathing, sleep apnea (a sleep disorder that causes breathing to stop for short periods during sleep), seizures, or depression. Also tell your doctor if you have ever thought about harming or killing yourself or planned or tried to do so, if you use or have ever used street drugs, or if you have overused prescription medications.If you experience any of the following symptoms, call your doctor immediately: depression, confusion, abnormal thoughts, thoughts of harming or killing yourself, anxiety, feeling that others want to harm you, hallucinations (seeing things or hearing voices that do not exist), loss of contact with reality, agitation, memory problems, breathing problems, snoring, sleep apnea, slowed or stopped breathing,or excessive drowsiness during the day.
Sodium oxybate is not available at retail pharmacies. A special program is in place to distribute the medication and provide information about the medication. You will receive written information and an instructional video about the safe use of sodium oxybate. Your medication will be mailed to you from a central pharmacy after you have read the information and talked to a pharmacist. Ask your doctor if you have any questions about how you will receive your medication.
Your doctor or pharmacist will give you the manufacturer's patient information sheet (Medication Guide) when you begin treatment with sodium oxybate and each time you refill your prescription. Read the information carefully and ask your doctor or pharmacist if you have any questions. You can also obtain the Medication Guide from the FDA website: http://www.fda.gov/cder/drug/infopage/xyrem/medicationguide.htm.
Keep all appointments with your doctor. You should see your doctor at least every 3 months.
Talk to your doctor about the risks of taking sodium oxybate.
Why is this medication prescribed
Sodium oxybate is used to prevent attacks of cataplexy (episodes of muscle weakness that begin suddenly and last for a short time) in patients who have narcolepsy (a sleep disorder that may cause extreme sleepiness, sudden uncontrollable urge to sleep during daily activities, and cataplexy). Sodium oxybate is in a class of medications called central nervous system depressants. The way that sodium oxybate works to treat cataplexy is not known.
How should this medicine be used
Sodium oxybate comes as a solution (liquid) to mix with water and take by mouth. It is usually taken twice each night because sodium oxybate wears off after a short time and the effects of one dose will not last for the entire night. The first dose is taken at bedtime, and a second dose is taken 2 1/2 to 4 hours after the first dose. Sodium oxybate must be taken on an empty stomach, so the first dose should be taken several hours after the evening meal. Try to allow the same amount of time between your evening meal and your first dose of sodium oxybate every night. Follow the directions on your prescription label carefully, and ask your doctor or pharmacist to explain any part you do not understand.
Do not take your bedtime dose of sodium oxybate until you are in bed and are ready to go to sleep for the night. Sodium oxybate begins to work very quickly and you may have an upset stomach or feel dizzy or lightheaded if you take the medication before you go to bed for the night.
Place your second dose of sodium oxybate in a safe place near your bed before you go to sleep. Use an alarm clock to be sure that you will wake up in time to take the second dose. If you wake up before the alarm goes off and it has been at least 2 1/2 hours since you took your first dose, take your second dose, turn off the alarm, and go back to sleep.
Your doctor will probably start you on a low dose of sodium oxybate and gradually increase your dose, not more often than once every 2 weeks.
Sodium oxybate may help to control your symptoms but will not cure your condition. Continue to take sodium oxybate even if you feel well. Do not stop taking sodium oxybate without talking to your doctor. Your doctor will probably want to decrease your dose gradually. If you suddenly stop taking sodium oxybate, you may have more attacks of cataplexy and you may experience anxiety and difficulty falling asleep or staying asleep.
To prepare doses of sodium oxybate, follow these steps:
- Open the carton that your medicine came in and remove the bottle of medication and the measuring device.
- Remove the measuring device from its wrapper.
- Open the bottle by pushing down on the cap and turning the cap counterclockwise (to the left) at the same time.
- Place the open bottle upright on a table.
- Hold the bottle upright with one hand. Use your other hand to place the tip of the measuring device in the center opening on the top of the bottle. Press the tip firmly into the opening.
- Hold the bottle and measuring device with one hand. Use your other hand to pull back on the plunger until it is even with the marking that matches the dose your doctor prescribed. Be sure to keep the bottle upright to allow the medication to flow into the measuring device.
- Remove the measuring device from the top of the bottle. Place the tip of the measuring device in one of the dosing cups provided with the medication.
- Press down on the plunger to empty the medication into the dosing cup.
- Add 2 ounces (60 mL, 1/4 cup, or about 4 tablespoons) of tap water to the dosing cup. The medication will taste best if you mix it with cold water. Do not mix the medication with fruit juice, soft drinks, or any other liquid.
- Repeat steps 5-9 to prepare a dose of sodium oxybate in the second dosing cup.
- Place the caps on both dosing cups. Turn each cap clockwise (to the right) until it clicks and locks in place.
- Rinse the measuring device with water
- Replace the cap on the bottle of sodium oxybate and return the bottle and measuring device to the safe place where they are stored. Place both prepared dosing cups of medication in a safe place near your bed.
- When it is time for you to take the first dose of sodium oxybate, press down on the cap and turn it counterclockwise (to the left). Drink all of the liquid while you are sitting on your bed. Put the cap back on the cup, turn it clockwise (to the right) to lock it in place, and lie down right away.
- When you wake up 2 1/2 - 4 hours later to take the second dose, repeat step 14.
Other uses for this medicine
This medication may be prescribed for other uses; ask your doctor or pharmacist for more information.
What special precautions should I follow
Before taking sodium oxybate:
- tell your doctor and pharmacist if you are allergic to sodium oxybate or any other medications.
- tell your doctor and pharmacist what other prescription and nonprescription medications, vitamins, nutritional supplements, and herbal products you are taking. Be sure to mention the medications listed in the IMPORTANT WARNING section and levodopa (Larodopa, in Sinemet). Your doctor may need to change the doses of your medications or monitor you carefully for side effects.
- tell your doctor if you are following a low salt diet for medical reasons and if you have or have ever had succinic semialdehyde dehydrogenase deficiency (an inherited condition in which certain substances build up in the body and cause retardation and developmental delays), heart failure, high blood pressure, or liver or kidney disease.
- tell your doctor if you are pregnant, plan to become pregnant, or are breast-feeding. If you become pregnant while taking sodium oxybate, call your doctor.
- if you are having surgery, including dental surgery, tell the doctor or dentist that you are taking sodium oxybate.
- you should know that you will be very sleepy for at least 6 hours after you take sodium oxybate, and you may also be drowsy during the daytime. Do not drive a car, operate machinery, or perform any other dangerous activities for at least 6 hours after you take your medication. Avoid dangerous activities at all times until you know how sodium oxybate affects you.
What special dietary instructions should I follow
Unless your doctor tells you otherwise, continue your normal diet.
What should I do if I forget a dose
If you miss the first dose of sodium oxybate, you may take a dose when the second dose is scheduled; do not take a second dose of sodium oxybate that night. If you miss the second dose, skip the missed dose and continue your regular dosing schedule on the next night. Do not take a double dose to make up for a missed one. Always allow at least 2 1/2 hours between doses of sodium oxybate.
Side effects
Mild side effects
Sodium oxybate may cause side effects. Tell your doctor if any of these symptoms are severe or do not go away:
- bedwetting
- headache
- dizziness
- upset stomach
- vomiting
- diarrhea
- heartburn
- stomach pain
- back pain
- weakness
- difficulty falling asleep or staying asleep
- sweating
- flu-like symptoms
- ringing in the ears
- problems with vision
- painful or irregular menstrual periods
- abnormal sensitivity to touch or sound
Severe side effects
Some side effects can be serious. The following symptoms are uncommon, but if you experience any of them or those listed in the IMPORTANT WARNING section, call your doctor immediately:
- sleepwalking
- abnormal dreams
- sore throat, fever, chills, and other signs of infection
Sodium oxybate may cause other side effects. Call your doctor if you have any unusual problems while taking this medication.
If you experience a serious side effect, you or your doctor may send a report to the Food and Drug Administration's (FDA) MedWatch Adverse Event Reporting program online [at http://www.fda.gov/MedWatch/report.htm] or by phone [1-800-332-1088].
What storage conditions are needed for this medicine
Keep this medication in the container it came in, tightly closed, and out of reach of children and pets. Store it at room temperature and away from excess heat and moisture (not in the bathroom). Throw away any medication that is outdated or no longer needed. Throw away unused mixtures of sodium oxybate and water 24 hours after you prepare them. When you are ready to throw away a bottle of sodium oxybate, pour any remaining medication down the drain, use a marker to destroy the bottle label, and throw away the bottle with your household trash. Ask your doctor or call the central pharmacy if you have questions about the proper disposal of your medication.
In case of emergency/overdose
In case of overdose, call your local poison control center at 1-800-222-1222. If the victim has collapsed or is not breathing, call local emergency services at 911.
Symptoms of overdose may include:
- confusion
- problems with coordination
- agitation
- loss of consciousness
- coma
- slow, shallow, or interrupted breathing
- loss of bladder control
- loss of bowel control
- vomiting
- sweating
- headache
- blurred vision
- muscle jerks or twitches
- seizure
- slow heartbeat
- low body temperature
- weak muscles
What other information should I know
Ask your doctor or call the central pharmacy if you have any questions about refilling your prescription.
Brand names
- Xyrem®
Other names
- Gamma Hydroxybutyrate Sodium
- GBH Sodium
- GHB Sodium
- γ-Hydroxybutyrate Sodium
- Oxybate Sodium
- Pages with script errors
- Pages with broken file links
- Chemical articles with multiple compound IDs
- Multiple chemicals in an infobox that need indexing
- Chemical articles with unknown parameter in Chembox
- Articles with changed EBI identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Chembox having DSD data
- Chembox having GHS data
- Articles containing unverified chemical infoboxes
- Chembox image size set
- Neurologic Drugs