Sodium monofluorophosphate: Difference between revisions
m (Robot: Automated text replacement (-{{reflist}} +{{reflist|2}}, -<references /> +{{reflist|2}}, -{{WikiDoc Cardiology Network Infobox}} +)) |
m (Protected "Sodium monofluorophosphate": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (10 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
|authorTag={{RB}} | |||
|OTC=Yes | |||
|genericName=Sodium monofluorophosphate | |||
|aOrAn=a | |||
|drugClass=OTC dental preaparation | |||
|indicationType=treatment | |||
|indication=cavities | |||
|adverseReactions=[[flatulence]] and [[bloating|abdominal bloating]] | |||
|blackBoxWarningTitle=<span style="color:#FF0000;">ConditionName: </span> | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
| | |fdaLIADAdult=====Indications==== | ||
| | * helps protect against cavities | ||
| | |||
| | ====Directions==== | ||
| | |||
| | : [[File:Sodium monoflurophosphate Directions.png|none|500px]] | ||
| | |offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| | |||
<!--Non–Guideline-Supported Use (Adult)--> | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
| | |||
| | <!--Pediatric Indications and Dosage--> | ||
| | |||
| | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
| | |fdaLIADPed=There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | ||
| | |||
| | <!--Off-Label Use and Dosage (Pediatric)--> | ||
| | <!--Guideline-Supported Use (Pediatric)--> | ||
| | |offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | ||
| | |||
| | <!--Non–Guideline-Supported Use (Pediatric)--> | ||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
}} | |||
{{ | <!--Contraindications--> | ||
|contraindications=<!--Warnings--> | |||
|warnings=* Keep out of the reach of children under 6 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials=[[flatulence]] and [[bloating|abdominal bloating]] | |||
<--Postmarketing Experience--> | |||
|postmarketing=There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
<--Drug Interactions--> | |||
|drugInteractions= | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA=* '''Pregnancy Category''' | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=There is no FDA guidance on the use of {{PAGENAME}} with respect to nursing mothers. | |||
|useInPed=There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | |||
|useInGeri=There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration=* Oral | |||
|monitoring=There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
<!--IV Compatibility--> | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose=* If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away. | |||
<!--Pharmacology--> | |||
<!--Drug box 2--> | |||
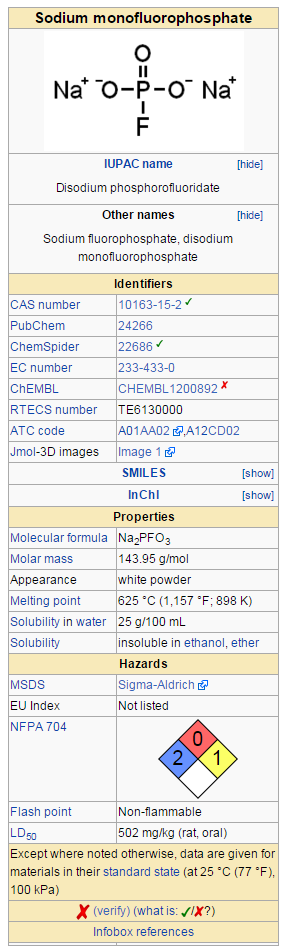
|drugBox=: [[File:Sodium monoflurophosphate Wiki.png|thumb|none|500px|This image is provided by Wikipedia]] | |||
<!--Mechanism of Action--> | |||
|mechAction= | |||
<!--Structure--> | |||
|structure======ACTIVE INGREDIENT===== | |||
* Sodium monofluorophosphate 0.76% (0.15% w/v fluoride ion) | |||
====INACTIVE INGREDIENTS==== | |||
* dicalcium phosphate dihydrate, water, glycerin, sodium lauryl sulfate, cellulose gum, flavor, tetrasodium pyrophosphate, sodium saccharin | |||
<!--Pharmacodynamics--> | |||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | |||
|PK=There is limited information regarding <i>Pharmacokinetics</i> of {{PAGENAME}} in the drug label. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
<!--Clinical Studies--> | |||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | |||
|howSupplied= | |||
|packLabel=====PRINCIPAL DISPLAY PANEL - 85 GRAM TUBE==== | |||
Colgate® | |||
Fluoride Toothpaste | |||
ADA | |||
Accepted | |||
American | |||
Dental | |||
Association® | |||
NET WT 3.0 OZ (85 g) | |||
Cavity | |||
Protection | |||
Strengthens Teeth | |||
with Active Fluoride | |||
Great Regular Flavor | |||
: [[File:Sodium monoflurophosphate PDP.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
====PRINCIPAL DISPLAY PANEL - 85 GRAM TUBE CARTON==== | |||
Colgate® | |||
Fluoride Toothpaste | |||
ADA | |||
Accepted | |||
American | |||
Dental | |||
Association® | |||
NET WT 3.0 OZ (85 g) | |||
Cavity | |||
Protection | |||
Strengthens Teeth | |||
with Active Fluoride | |||
Great Regular Flavor | |||
: [[File:Sodium monoflurophosphate PDP 2.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
====Ingredients and Appearance==== | |||
: [[File:Sodium monoflurophosphate Ing and App.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo=====QUESTIONS OR COMMENTS?==== | |||
* Call toll-free 1-800-468-6502 | |||
<!--Precautions with Alcohol--> | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames=* COLGATE TOOTHPASTE GREAT REGULAR FLAVOR®<ref>{{Cite web | title = sodium monofluorophosphate | url =http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=ce5c6085-718d-409b-8d0f-e2e8419016c4 }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
|lookAlike=<!--Drug Shortage Status--> | |||
|drugShortage= | |||
}} | |||
<!--Pill Image--> | |||
<!--Label Display Image--> | |||
<!--Category--> | |||
< | |||
[[Category:Drug]] | |||
[[Category: | |||
Latest revision as of 17:09, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
NOTE: Most over the counter (OTC) are not reviewed and approved by the FDA. However, they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Overview
Sodium monofluorophosphate is a OTC dental preaparation that is FDA approved for the treatment of cavities. Common adverse reactions include flatulence and abdominal bloating.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
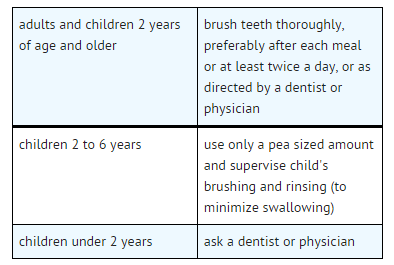
Indications
- helps protect against cavities
Directions
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sodium monofluorophosphate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sodium monofluorophosphate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Sodium monofluorophosphate in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Sodium monofluorophosphate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Sodium monofluorophosphate in pediatric patients.
Contraindications
There is limited information regarding Sodium monofluorophosphate Contraindications in the drug label.
Warnings
- Keep out of the reach of children under 6 years of age. If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Adverse Reactions
Clinical Trials Experience
flatulence and abdominal bloating
<--Postmarketing Experience-->
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Sodium monofluorophosphate in the drug label.
<--Drug Interactions-->
Drug Interactions
There is limited information regarding Sodium monofluorophosphate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Sodium monofluorophosphate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Sodium monofluorophosphate during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Sodium monofluorophosphate with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Sodium monofluorophosphate with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Sodium monofluorophosphate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Sodium monofluorophosphate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Sodium monofluorophosphate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Sodium monofluorophosphate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Sodium monofluorophosphate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sodium monofluorophosphate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sodium monofluorophosphate in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
There is limited information regarding Monitoring of Sodium monofluorophosphate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Sodium monofluorophosphate in the drug label.
Overdosage
- If more than used for brushing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
Pharmacology
Mechanism of Action
There is limited information regarding Sodium monofluorophosphate Mechanism of Action in the drug label.
Structure
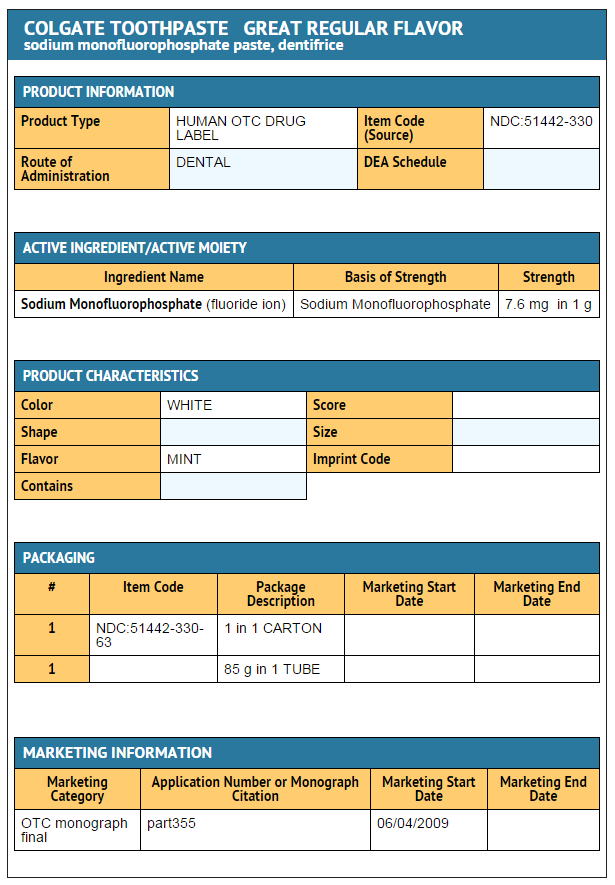
ACTIVE INGREDIENT
- Sodium monofluorophosphate 0.76% (0.15% w/v fluoride ion)
INACTIVE INGREDIENTS
- dicalcium phosphate dihydrate, water, glycerin, sodium lauryl sulfate, cellulose gum, flavor, tetrasodium pyrophosphate, sodium saccharin
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Sodium monofluorophosphate in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Sodium monofluorophosphate in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Sodium monofluorophosphate in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Sodium monofluorophosphate in the drug label.
How Supplied
There is limited information regarding Sodium monofluorophosphate How Supplied in the drug label.
Storage
There is limited information regarding Sodium monofluorophosphate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Sodium monofluorophosphate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL - 85 GRAM TUBE
Colgate® Fluoride Toothpaste
ADA Accepted American Dental Association®
NET WT 3.0 OZ (85 g)
Cavity Protection
Strengthens Teeth with Active Fluoride
Great Regular Flavor
PRINCIPAL DISPLAY PANEL - 85 GRAM TUBE CARTON
Colgate® Fluoride Toothpaste
ADA Accepted American Dental Association®
NET WT 3.0 OZ (85 g)
Cavity Protection
Strengthens Teeth with Active Fluoride
Great Regular Flavor
Ingredients and Appearance
{{#ask: Label Page::Sodium monofluorophosphate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
QUESTIONS OR COMMENTS?
- Call toll-free 1-800-468-6502
Precautions with Alcohol
- Alcohol-Sodium monofluorophosphate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- COLGATE TOOTHPASTE GREAT REGULAR FLAVOR®[1]
Look-Alike Drug Names
There is limited information regarding Sodium monofluorophosphate Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.