Saxagliptin: Difference between revisions
m (Robot: Automated text replacement (-{{reflist}} +{{reflist|2}}, -<references /> +{{reflist|2}}, -{{WikiDoc Cardiology Network Infobox}} +)) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
|authorTag= | |||
{{VP}} | |||
{{ | <!--Overview--> | ||
| | |||
| | |genericName= | ||
| | |||
| | Saxagliptin | ||
| | |||
| | |aOrAn= | ||
| | |||
| | a | ||
| | |||
| | |drugClass= | ||
| | |||
| | [[dipeptidyl peptidase-4]] (DPP4) inhibitor | ||
| | |||
| | |indication= | ||
| | |||
| | [[type 2 diabetes mellitus]] | ||
| | |||
|hasBlackBoxWarning= | |||
|adverseReactions= | |||
[[upper respiratory tract infection]], [[urinary tract infection]], and [[headache]] | |||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle= | |||
Title | |||
|blackBoxWarningBody= | |||
<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
|fdaLIADAdult= | |||
=====Type 2 Diabetes Mellitus===== | |||
*Monotherapy and Combination Therapy | |||
:*ONGLYZA is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus in multiple clinical settings. | |||
:*The recommended dosage of ONGLYZA is 2.5 mg or 5 mg once daily taken regardless of meals. | |||
<!--Off-Label Use and Dosage (Adult)--> | |||
<!--Guideline-Supported Use (Adult)--> | |||
|offLabelAdultGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | |||
|offLabelAdultNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | |||
|fdaLIADPed= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | |||
<!--Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications= | |||
*ONGLYZA is contraindicated in patients with a history of a serious [[hypersensitivity]] reaction to ONGLYZA, such as [[anaphylaxis]], [[angioedema]], or [[exfoliative]] skin conditions. | |||
<!--Warnings--> | |||
|warnings= | |||
====Precautions==== | |||
*[[Pancreatitis]] | |||
:*There have been postmarketing reports of acute [[pancreatitis]] in patients taking ONGLYZA. After initiation of ONGLYZA, patients should be observed carefully for signs and symptoms of [[pancreatitis]]. If [[pancreatitis]] is suspected, ONGLYZA should promptly be discontinued and appropriate management should be initiated. It is unknown whether patients with a history of [[pancreatitis]] are at increased risk for the development of [[pancreatitis]] while using ONGLYZA. | |||
*[[Hypoglycemia]] with Cocomitant Use of Sulfonylurea or Insulin | |||
:*When ONGLYZA was used in combination with a sulfonylurea or with insulin, medications known to cause [[hypoglycemia]], the incidence of confirmed [[hypoglycemia]] was increased over that of placebo used in combination with a [[sulfonylurea]] or with [[insulin]]. Therefore, a lower dose of the [[insulin]] secretagogue or insulin may be required to minimize the risk of [[hypoglycemia]] when used in combination with ONGLYZA. | |||
*Hypersensitivity Reactions | |||
:*There have been postmarketing reports of serious [[hypersensitivity]] reactions in patients treated with ONGLYZA. These reactions include [[anaphylaxis]], [[angioedema]], and [[exfoliative]] skin conditions. Onset of these reactions occurred within the first 3 months after initiation of treatment with ONGLYZA, with some reports occurring after the first dose. If a serious [[hypersensitivity]] reaction is suspected, discontinue ONGLYZA, assess for other potential causes for the event, and institute alternative treatment for diabetes. | |||
:*Use caution in a patient with a history of [[angioedema]] to another [[dipeptidyl peptidase-4]] (DPP4) inhibitor because it is unknown whether such patients will be predisposed to angioedema with ONGLYZA. | |||
*Macrovascular Outcomes | |||
:*There have been no clinical studies establishing conclusive evidence of [[macrovascular]] risk reduction with ONGLYZA or any other [[antidiabetic]] drug. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
*Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. | |||
=====Adverse Reactions with Monotherapy and with Add-On Combination Therapy===== | |||
*In two placebo-controlled monotherapy trials of 24-weeks duration, patients were treated with ONGLYZA 2.5 mg daily, ONGLYZA 5 mg daily, and placebo. Three 24-week, placebo-controlled, add-on combination therapy trials were also conducted: one with metformin, one with a thiazolidinedione (pioglitazone or rosiglitazone), and one with glyburide. In these three trials, patients were randomized to add-on therapy with ONGLYZA 2.5 mg daily, ONGLYZA 5 mg daily, or placebo. A saxagliptin 10 mg treatment arm was included in one of the monotherapy trials and in the add-on combination trial with metformin. The 10 mg dosage is not an approved dosage. | |||
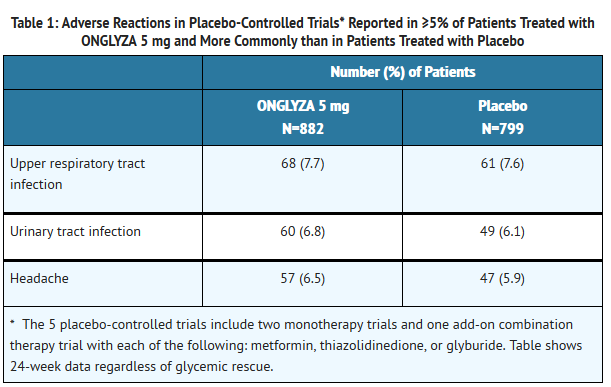
*In a prespecified pooled analysis of the 24-week data (regardless of glycemic rescue) from the two monotherapy trials, the add-on to metformin trial, the add-on to thiazolidinedione (TZD) trial, and the add-on to glyburide trial, the overall incidence of adverse events in patients treated with ONGLYZA 2.5 mg and ONGLYZA 5 mg was similar to placebo (72% and 72.2% versus 70.6%, respectively). Discontinuation of therapy due to adverse events occurred in 2.2%, 3.3%, and 1.8% of patients receiving ONGLYZA 2.5 mg, ONGLYZA 5 mg, and placebo, respectively. The most common adverse events (reported in at least 2 patients treated with ONGLYZA 2.5 mg or at least 2 patients treated with ONGLYZA 5 mg) associated with premature discontinuation of therapy included lymphopenia (0.1% and 0.5% versus 0%, respectively), rash (0.2% and 0.3% versus 0.3%), blood creatinine increased (0.3% and 0% versus 0%), and blood creatine phosphokinase increased (0.1% and 0.2% versus 0%). The adverse reactions in this pooled analysis reported (regardless of investigator assessment of causality) in ≥5% of patients treated with ONGLYZA 5 mg, and more commonly than in patients treated with placebo are shown in Table 1. | |||
T1 | |||
*In patients treated with ONGLYZA 2.5 mg, headache (6.5%) was the only adverse reaction reported at a rate ≥5% and more commonly than in patients treated with placebo. | |||
*In this pooled analysis, adverse reactions that were reported in ≥2% of patients treated with ONGLYZA 2.5 mg or ONGLYZA 5 mg and ≥1% more frequently compared to placebo included: sinusitis (2.9% and 2.6% versus 1.6%, respectively), abdominal pain (2.4% and 1.7% versus 0.5%), gastroenteritis (1.9% and 2.3% versus 0.9%), and vomiting (2.2% and 2.3% versus 1.3%). | |||
*In the add-on to TZD trial, the incidence of peripheral edema was higher for ONGLYZA 5 mg versus placebo (8.1% and 4.3%, respectively). The incidence of peripheral edema for ONGLYZA 2.5 mg was 3.1%. None of the reported adverse reactions of peripheral edema resulted in study drug discontinuation. Rates of peripheral edema for ONGLYZA 2.5 mg and ONGLYZA 5 mg versus placebo were 3.6% and 2% versus 3% given as monotherapy, 2.1% and 2.1% versus 2.2% given as add-on therapy to metformin, and 2.4% and 1.2% versus 2.2% given as add-on therapy to glyburide. | |||
*The incidence rate of fractures was 1.0 and 0.6 per 100 patient-years, respectively, for ONGLYZA (pooled analysis of 2.5 mg, 5 mg, and 10 mg) and placebo. The 10 mg dosage is not an approved dosage. The incidence rate of fracture events in patients who received ONGLYZA did not increase over time. Causality has not been established and nonclinical studies have not demonstrated adverse effects of ONGLYZA on bone. | |||
*An event of thrombocytopenia, consistent with a diagnosis of idiopathic thrombocytopenic purpura, was observed in the clinical program. The relationship of this event to ONGLYZA is not known. | |||
=====Adverse Reactions in Patients with Renal Impairment===== | |||
*ONGLYZA 2.5 mg was compared to placebo in a 12-week trial in 170 patients with type 2 diabetes and moderate or severe renal impairment or end-stage renal disease (ESRD). The incidence of adverse events, including serious adverse events and discontinuations due to adverse events, was similar between ONGLYZA and placebo. | |||
======Adverse Reactions with Concomitant Use with Insulin===== | |||
*In the add-on to insulin trial [see Clinical Studies (14.2)], the incidence of adverse events, including serious adverse events and discontinuations due to adverse events, was similar between ONGLYZA and placebo, except for confirmed hypoglycemia [See Adverse Reactions (6.1)]. | |||
=====Adverse Reactions with Concomitant Use with Metformin in Treatment-Naive Patients with Type 2 Diabetes===== | |||
*Table 2 shows the adverse reactions reported (regardless of investigator assessment of causality) in ≥5% of patients participating in an additional 24-week, active-controlled trial of coadministered ONGLYZA and metformin in treatment-naive patients. | |||
=====Hypoglycemia===== | |||
*Adverse reactions of hypoglycemia were based on all reports of hypoglycemia. A concurrent glucose measurement was not required or was normal in some patients. Therefore, it is not possible to conclusively determine that all these reports reflect true hypoglycemia. | |||
*In the add-on to glyburide study, the overall incidence of reported hypoglycemia was higher for ONGLYZA 2.5 mg and ONGLYZA 5 mg (13.3% and 14.6%) versus placebo (10.1%). The incidence of confirmed hypoglycemia in this study, defined as symptoms of hypoglycemia accompanied by a fingerstick glucose value of ≤50 mg/dL, was 2.4% and 0.8% for ONGLYZA 2.5 mg and ONGLYZA 5 mg and 0.7% for placebo [see Warnings and Precautions (5.2)]. The incidence of reported hypoglycemia for ONGLYZA 2.5 mg and ONGLYZA 5 mg versus placebo given as monotherapy was 4% and 5.6% versus 4.1%, respectively, 7.8% and 5.8% versus 5% given as add-on therapy to metformin, and 4.1% and 2.7% versus 3.8% given as add-on therapy to TZD. The incidence of reported hypoglycemia was 3.4% in treatment-naive patients given ONGLYZA 5 mg plus metformin and 4% in patients given metformin alone. | |||
*In the active-controlled trial comparing add-on therapy with ONGLYZA 5 mg to glipizide in patients inadequately controlled on metformin alone, the incidence of reported hypoglycemia was 3% (19 events in 13 patients) with ONGLYZA 5 mg versus 36.3% (750 events in 156 patients) with glipizide. Confirmed symptomatic hypoglycemia (accompanying fingerstick blood glucose ≤50 mg/dL) was reported in none of the ONGLYZA-treated patients and in 35 glipizide-treated patients (8.1%) (p<0.0001). | |||
*During 12 weeks of treatment in patients with moderate or severe renal impairment or ESRD, the overall incidence of reported hypoglycemia was 20% among patients treated with ONGLYZA 2.5 mg and 22% among patients treated with placebo. Four ONGLYZA-treated patients (4.7%) and three placebo-treated patients (3.5%) reported at least one episode of confirmed symptomatic hypoglycemia (accompanying fingerstick glucose ≤50 mg/dL). | |||
*In the add-on to insulin trial, the overall incidence of reported hypoglycemia was 18.4% for ONGLYZA 5 mg and 19.9% for placebo. However, the incidence of confirmed symptomatic hypoglycemia (accompanying fingerstick blood glucose ≤50 mg/dL) was higher with ONGLYZA 5 mg (5.3%) versus placebo (3.3%). | |||
*In the add-on to metformin plus sulfonylurea trial, the overall incidence of reported hypoglycemia was 10.1% for ONGLYZA 5 mg and 6.3% for placebo. Confirmed hypoglycemia was reported in 1.6% of the ONGLYZA-treated patients and in none of the placebo-treated patients [see Warnings and Precautions (5.2)]. | |||
=====Hypersensitivity Reactions===== | |||
*Hypersensitivity-related events, such as urticaria and facial edema in the 5-study pooled analysis up to Week 24 were reported in 1.5%, 1.5%, and 0.4% of patients who received ONGLYZA 2.5 mg, ONGLYZA 5 mg, and placebo, respectively. None of these events in patients who received ONGLYZA required hospitalization or were reported as life-threatening by the investigators. One ONGLYZA-treated patient in this pooled analysis discontinued due to generalized urticaria and facial edema. | |||
=====Infections===== | |||
*In the unblinded, controlled, clinical trial database for ONGLYZA to date, there have been 6 (0.12%) reports of tuberculosis among the 4959 ONGLYZA-treated patients (1.1 per 1000 patient-years) compared to no reports of tuberculosis among the 2868 comparator-treated patients. Two of these six cases were confirmed with laboratory testing. The remaining cases had limited information or had presumptive diagnoses of tuberculosis. None of the six cases occurred in the United States or in Western Europe. One case occurred in Canada in a patient originally from Indonesia who had recently visited Indonesia. The duration of treatment with ONGLYZA until report of tuberculosis ranged from 144 to 929 days. Post-treatment lymphocyte counts were consistently within the reference range for four cases. One patient had lymphopenia prior to initiation of ONGLYZA that remained stable throughout ONGLYZA treatment. The final patient had an isolated lymphocyte count below normal approximately four months prior to the report of tuberculosis. There have been no spontaneous reports of tuberculosis associated with ONGLYZA use. Causality has not been estimated and there are too few cases to date to determine whether tuberculosis is related to ONGLYZA use. | |||
*There has been one case of a potential opportunistic infection in the unblinded, controlled clinical trial database to date in an ONGLYZA-treated patient who developed suspected foodborne fatal salmonella sepsis after approximately 600 days of ONGLYZA therapy. There have been no spontaneous reports of opportunistic infections associated with ONGLYZA use. | |||
=====Vital Signs===== | |||
*No clinically meaningful changes in vital signs have been observed in patients treated with ONGLYZA. | |||
=====Laboratory Tests===== | |||
=====Absolute Lymphocyte Counts===== | |||
*There was a dose-related mean decrease in absolute lymphocyte count observed with ONGLYZA. From a baseline mean absolute lymphocyte count of approximately 2200 cells/microL, mean decreases of approximately 100 and 120 cells/microL with ONGLYZA 5 mg and 10 mg, respectively, relative to placebo were observed at 24 weeks in a pooled analysis of five placebo-controlled clinical studies. Similar effects were observed when ONGLYZA 5 mg was given in initial combination with metformin compared to metformin alone. There was no difference observed for ONGLYZA 2.5 mg relative to placebo. The proportion of patients who were reported to have a lymphocyte count ≤750 cells/microL was 0.5%, 1.5%, 1.4%, and 0.4% in the ONGLYZA 2.5 mg, 5 mg, 10 mg, and placebo groups, respectively. In most patients, recurrence was not observed with repeated exposure to ONGLYZA although some patients had recurrent decreases upon rechallenge that led to discontinuation of ONGLYZA. The decreases in lymphocyte count were not associated with clinically relevant adverse reactions. The 10 mg dosage is not an approved dosage. | |||
*The clinical significance of this decrease in lymphocyte count relative to placebo is not known. When clinically indicated, such as in settings of unusual or prolonged infection, lymphocyte count should be measured. The effect of ONGLYZA on lymphocyte counts in patients with lymphocyte abnormalities (e.g., human immunodeficiency virus) is unknown. | |||
<!--Postmarketing Experience--> | |||
|postmarketing= | |||
*Additional adverse reactions have been identified during postapproval use of ONGLYZA. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure. | |||
:*Hypersensitivity reactions including [[anaphylaxis]], angioedema, and exfoliative skin conditions. | |||
:*Acute pancreatitis. | |||
|drugInteractions= | |||
*Strong Inhibitors of CYP3A4/5 Enzymes | |||
:*Ketoconazole significantly increased saxagliptin exposure. Similar significant increases in plasma concentrations of saxagliptin are anticipated with other strong CYP3A4/5 inhibitors (e.g., atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, and telithromycin). The dose of ONGLYZA should be limited to 2.5 mg when coadministered with a strong CYP3A4/5 inhibitor. | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category''' | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery= | |||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to nursing mothers. | |||
|useInPed= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | |||
|useInGeri= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration= | |||
* Oral | |||
* Intravenous | |||
|monitoring= | |||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
* Description | |||
<!--IV Compatibility--> | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose= | |||
===Acute Overdose=== | |||
====Signs and Symptoms==== | |||
* Description | |||
====Management==== | |||
* Description | |||
===Chronic Overdose=== | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacology--> | |||
<!--Drug box 2--> | |||
|drugBox= | |||
<!--Mechanism of Action--> | |||
|mechAction= | |||
* | |||
<!--Structure--> | |||
|structure= | |||
* | |||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Pharmacodynamics--> | |||
|PD= | |||
There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | |||
|PK= | |||
There is limited information regarding <i>Pharmacokinetics</i> of {{PAGENAME}} in the drug label. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic= | |||
There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
<!--Clinical Studies--> | |||
|clinicalStudies= | |||
There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | |||
|howSupplied= | |||
* | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo= | |||
There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
<!--Precautions with Alcohol--> | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames= | |||
* ®<ref>{{Cite web | title = | url = }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
|lookAlike= | |||
* A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
<!--Drug Shortage Status--> | |||
|drugShortage= | |||
}} | }} | ||
<!--Pill Image--> | |||
{{PillImage | |||
|fileName=No image.jpg|This image is provided by the National Library of Medicine. | |||
|drugName= | |||
|NDC= | |||
|drugAuthor= | |||
|ingredients= | |||
|pillImprint= | |||
|dosageValue= | |||
|dosageUnit= | |||
|pillColor= | |||
|pillShape= | |||
|pillSize= | |||
|pillScore= | |||
}} | |||
<!--Label Display Image--> | |||
= | {{LabelImage | ||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
= | |||
<!--Category--> | |||
[[Category:Drug]] | |||
Revision as of 20:58, 7 November 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Saxagliptin is a dipeptidyl peptidase-4 (DPP4) inhibitor that is FDA approved for the {{{indicationType}}} of type 2 diabetes mellitus. Common adverse reactions include upper respiratory tract infection, urinary tract infection, and headache.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Type 2 Diabetes Mellitus
- Monotherapy and Combination Therapy
- ONGLYZA is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus in multiple clinical settings.
- The recommended dosage of ONGLYZA is 2.5 mg or 5 mg once daily taken regardless of meals.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Saxagliptin in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Saxagliptin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Saxagliptin in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Saxagliptin in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Saxagliptin in pediatric patients.
Contraindications
- ONGLYZA is contraindicated in patients with a history of a serious hypersensitivity reaction to ONGLYZA, such as anaphylaxis, angioedema, or exfoliative skin conditions.
Warnings
Precautions
- There have been postmarketing reports of acute pancreatitis in patients taking ONGLYZA. After initiation of ONGLYZA, patients should be observed carefully for signs and symptoms of pancreatitis. If pancreatitis is suspected, ONGLYZA should promptly be discontinued and appropriate management should be initiated. It is unknown whether patients with a history of pancreatitis are at increased risk for the development of pancreatitis while using ONGLYZA.
- Hypoglycemia with Cocomitant Use of Sulfonylurea or Insulin
- When ONGLYZA was used in combination with a sulfonylurea or with insulin, medications known to cause hypoglycemia, the incidence of confirmed hypoglycemia was increased over that of placebo used in combination with a sulfonylurea or with insulin. Therefore, a lower dose of the insulin secretagogue or insulin may be required to minimize the risk of hypoglycemia when used in combination with ONGLYZA.
- Hypersensitivity Reactions
- There have been postmarketing reports of serious hypersensitivity reactions in patients treated with ONGLYZA. These reactions include anaphylaxis, angioedema, and exfoliative skin conditions. Onset of these reactions occurred within the first 3 months after initiation of treatment with ONGLYZA, with some reports occurring after the first dose. If a serious hypersensitivity reaction is suspected, discontinue ONGLYZA, assess for other potential causes for the event, and institute alternative treatment for diabetes.
- Use caution in a patient with a history of angioedema to another dipeptidyl peptidase-4 (DPP4) inhibitor because it is unknown whether such patients will be predisposed to angioedema with ONGLYZA.
- Macrovascular Outcomes
- There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with ONGLYZA or any other antidiabetic drug.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions with Monotherapy and with Add-On Combination Therapy
- In two placebo-controlled monotherapy trials of 24-weeks duration, patients were treated with ONGLYZA 2.5 mg daily, ONGLYZA 5 mg daily, and placebo. Three 24-week, placebo-controlled, add-on combination therapy trials were also conducted: one with metformin, one with a thiazolidinedione (pioglitazone or rosiglitazone), and one with glyburide. In these three trials, patients were randomized to add-on therapy with ONGLYZA 2.5 mg daily, ONGLYZA 5 mg daily, or placebo. A saxagliptin 10 mg treatment arm was included in one of the monotherapy trials and in the add-on combination trial with metformin. The 10 mg dosage is not an approved dosage.
- In a prespecified pooled analysis of the 24-week data (regardless of glycemic rescue) from the two monotherapy trials, the add-on to metformin trial, the add-on to thiazolidinedione (TZD) trial, and the add-on to glyburide trial, the overall incidence of adverse events in patients treated with ONGLYZA 2.5 mg and ONGLYZA 5 mg was similar to placebo (72% and 72.2% versus 70.6%, respectively). Discontinuation of therapy due to adverse events occurred in 2.2%, 3.3%, and 1.8% of patients receiving ONGLYZA 2.5 mg, ONGLYZA 5 mg, and placebo, respectively. The most common adverse events (reported in at least 2 patients treated with ONGLYZA 2.5 mg or at least 2 patients treated with ONGLYZA 5 mg) associated with premature discontinuation of therapy included lymphopenia (0.1% and 0.5% versus 0%, respectively), rash (0.2% and 0.3% versus 0.3%), blood creatinine increased (0.3% and 0% versus 0%), and blood creatine phosphokinase increased (0.1% and 0.2% versus 0%). The adverse reactions in this pooled analysis reported (regardless of investigator assessment of causality) in ≥5% of patients treated with ONGLYZA 5 mg, and more commonly than in patients treated with placebo are shown in Table 1.
T1
- In patients treated with ONGLYZA 2.5 mg, headache (6.5%) was the only adverse reaction reported at a rate ≥5% and more commonly than in patients treated with placebo.
- In this pooled analysis, adverse reactions that were reported in ≥2% of patients treated with ONGLYZA 2.5 mg or ONGLYZA 5 mg and ≥1% more frequently compared to placebo included: sinusitis (2.9% and 2.6% versus 1.6%, respectively), abdominal pain (2.4% and 1.7% versus 0.5%), gastroenteritis (1.9% and 2.3% versus 0.9%), and vomiting (2.2% and 2.3% versus 1.3%).
- In the add-on to TZD trial, the incidence of peripheral edema was higher for ONGLYZA 5 mg versus placebo (8.1% and 4.3%, respectively). The incidence of peripheral edema for ONGLYZA 2.5 mg was 3.1%. None of the reported adverse reactions of peripheral edema resulted in study drug discontinuation. Rates of peripheral edema for ONGLYZA 2.5 mg and ONGLYZA 5 mg versus placebo were 3.6% and 2% versus 3% given as monotherapy, 2.1% and 2.1% versus 2.2% given as add-on therapy to metformin, and 2.4% and 1.2% versus 2.2% given as add-on therapy to glyburide.
- The incidence rate of fractures was 1.0 and 0.6 per 100 patient-years, respectively, for ONGLYZA (pooled analysis of 2.5 mg, 5 mg, and 10 mg) and placebo. The 10 mg dosage is not an approved dosage. The incidence rate of fracture events in patients who received ONGLYZA did not increase over time. Causality has not been established and nonclinical studies have not demonstrated adverse effects of ONGLYZA on bone.
- An event of thrombocytopenia, consistent with a diagnosis of idiopathic thrombocytopenic purpura, was observed in the clinical program. The relationship of this event to ONGLYZA is not known.
Adverse Reactions in Patients with Renal Impairment
- ONGLYZA 2.5 mg was compared to placebo in a 12-week trial in 170 patients with type 2 diabetes and moderate or severe renal impairment or end-stage renal disease (ESRD). The incidence of adverse events, including serious adverse events and discontinuations due to adverse events, was similar between ONGLYZA and placebo.
=Adverse Reactions with Concomitant Use with Insulin
- In the add-on to insulin trial [see Clinical Studies (14.2)], the incidence of adverse events, including serious adverse events and discontinuations due to adverse events, was similar between ONGLYZA and placebo, except for confirmed hypoglycemia [See Adverse Reactions (6.1)].
Adverse Reactions with Concomitant Use with Metformin in Treatment-Naive Patients with Type 2 Diabetes
- Table 2 shows the adverse reactions reported (regardless of investigator assessment of causality) in ≥5% of patients participating in an additional 24-week, active-controlled trial of coadministered ONGLYZA and metformin in treatment-naive patients.
Hypoglycemia
- Adverse reactions of hypoglycemia were based on all reports of hypoglycemia. A concurrent glucose measurement was not required or was normal in some patients. Therefore, it is not possible to conclusively determine that all these reports reflect true hypoglycemia.
- In the add-on to glyburide study, the overall incidence of reported hypoglycemia was higher for ONGLYZA 2.5 mg and ONGLYZA 5 mg (13.3% and 14.6%) versus placebo (10.1%). The incidence of confirmed hypoglycemia in this study, defined as symptoms of hypoglycemia accompanied by a fingerstick glucose value of ≤50 mg/dL, was 2.4% and 0.8% for ONGLYZA 2.5 mg and ONGLYZA 5 mg and 0.7% for placebo [see Warnings and Precautions (5.2)]. The incidence of reported hypoglycemia for ONGLYZA 2.5 mg and ONGLYZA 5 mg versus placebo given as monotherapy was 4% and 5.6% versus 4.1%, respectively, 7.8% and 5.8% versus 5% given as add-on therapy to metformin, and 4.1% and 2.7% versus 3.8% given as add-on therapy to TZD. The incidence of reported hypoglycemia was 3.4% in treatment-naive patients given ONGLYZA 5 mg plus metformin and 4% in patients given metformin alone.
- In the active-controlled trial comparing add-on therapy with ONGLYZA 5 mg to glipizide in patients inadequately controlled on metformin alone, the incidence of reported hypoglycemia was 3% (19 events in 13 patients) with ONGLYZA 5 mg versus 36.3% (750 events in 156 patients) with glipizide. Confirmed symptomatic hypoglycemia (accompanying fingerstick blood glucose ≤50 mg/dL) was reported in none of the ONGLYZA-treated patients and in 35 glipizide-treated patients (8.1%) (p<0.0001).
- During 12 weeks of treatment in patients with moderate or severe renal impairment or ESRD, the overall incidence of reported hypoglycemia was 20% among patients treated with ONGLYZA 2.5 mg and 22% among patients treated with placebo. Four ONGLYZA-treated patients (4.7%) and three placebo-treated patients (3.5%) reported at least one episode of confirmed symptomatic hypoglycemia (accompanying fingerstick glucose ≤50 mg/dL).
- In the add-on to insulin trial, the overall incidence of reported hypoglycemia was 18.4% for ONGLYZA 5 mg and 19.9% for placebo. However, the incidence of confirmed symptomatic hypoglycemia (accompanying fingerstick blood glucose ≤50 mg/dL) was higher with ONGLYZA 5 mg (5.3%) versus placebo (3.3%).
- In the add-on to metformin plus sulfonylurea trial, the overall incidence of reported hypoglycemia was 10.1% for ONGLYZA 5 mg and 6.3% for placebo. Confirmed hypoglycemia was reported in 1.6% of the ONGLYZA-treated patients and in none of the placebo-treated patients [see Warnings and Precautions (5.2)].
Hypersensitivity Reactions
- Hypersensitivity-related events, such as urticaria and facial edema in the 5-study pooled analysis up to Week 24 were reported in 1.5%, 1.5%, and 0.4% of patients who received ONGLYZA 2.5 mg, ONGLYZA 5 mg, and placebo, respectively. None of these events in patients who received ONGLYZA required hospitalization or were reported as life-threatening by the investigators. One ONGLYZA-treated patient in this pooled analysis discontinued due to generalized urticaria and facial edema.
Infections
- In the unblinded, controlled, clinical trial database for ONGLYZA to date, there have been 6 (0.12%) reports of tuberculosis among the 4959 ONGLYZA-treated patients (1.1 per 1000 patient-years) compared to no reports of tuberculosis among the 2868 comparator-treated patients. Two of these six cases were confirmed with laboratory testing. The remaining cases had limited information or had presumptive diagnoses of tuberculosis. None of the six cases occurred in the United States or in Western Europe. One case occurred in Canada in a patient originally from Indonesia who had recently visited Indonesia. The duration of treatment with ONGLYZA until report of tuberculosis ranged from 144 to 929 days. Post-treatment lymphocyte counts were consistently within the reference range for four cases. One patient had lymphopenia prior to initiation of ONGLYZA that remained stable throughout ONGLYZA treatment. The final patient had an isolated lymphocyte count below normal approximately four months prior to the report of tuberculosis. There have been no spontaneous reports of tuberculosis associated with ONGLYZA use. Causality has not been estimated and there are too few cases to date to determine whether tuberculosis is related to ONGLYZA use.
- There has been one case of a potential opportunistic infection in the unblinded, controlled clinical trial database to date in an ONGLYZA-treated patient who developed suspected foodborne fatal salmonella sepsis after approximately 600 days of ONGLYZA therapy. There have been no spontaneous reports of opportunistic infections associated with ONGLYZA use.
Vital Signs
- No clinically meaningful changes in vital signs have been observed in patients treated with ONGLYZA.
Laboratory Tests
Absolute Lymphocyte Counts
- There was a dose-related mean decrease in absolute lymphocyte count observed with ONGLYZA. From a baseline mean absolute lymphocyte count of approximately 2200 cells/microL, mean decreases of approximately 100 and 120 cells/microL with ONGLYZA 5 mg and 10 mg, respectively, relative to placebo were observed at 24 weeks in a pooled analysis of five placebo-controlled clinical studies. Similar effects were observed when ONGLYZA 5 mg was given in initial combination with metformin compared to metformin alone. There was no difference observed for ONGLYZA 2.5 mg relative to placebo. The proportion of patients who were reported to have a lymphocyte count ≤750 cells/microL was 0.5%, 1.5%, 1.4%, and 0.4% in the ONGLYZA 2.5 mg, 5 mg, 10 mg, and placebo groups, respectively. In most patients, recurrence was not observed with repeated exposure to ONGLYZA although some patients had recurrent decreases upon rechallenge that led to discontinuation of ONGLYZA. The decreases in lymphocyte count were not associated with clinically relevant adverse reactions. The 10 mg dosage is not an approved dosage.
- The clinical significance of this decrease in lymphocyte count relative to placebo is not known. When clinically indicated, such as in settings of unusual or prolonged infection, lymphocyte count should be measured. The effect of ONGLYZA on lymphocyte counts in patients with lymphocyte abnormalities (e.g., human immunodeficiency virus) is unknown.
Postmarketing Experience
- Additional adverse reactions have been identified during postapproval use of ONGLYZA. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Hypersensitivity reactions including anaphylaxis, angioedema, and exfoliative skin conditions.
- Acute pancreatitis.
Drug Interactions
- Strong Inhibitors of CYP3A4/5 Enzymes
- Ketoconazole significantly increased saxagliptin exposure. Similar significant increases in plasma concentrations of saxagliptin are anticipated with other strong CYP3A4/5 inhibitors (e.g., atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, ritonavir, saquinavir, and telithromycin). The dose of ONGLYZA should be limited to 2.5 mg when coadministered with a strong CYP3A4/5 inhibitor.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Saxagliptin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Saxagliptin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Saxagliptin with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Saxagliptin with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Saxagliptin with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Saxagliptin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Saxagliptin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Saxagliptin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Saxagliptin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Saxagliptin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Saxagliptin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Saxagliptin in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Saxagliptin in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Saxagliptin in the drug label.
Pharmacology
There is limited information regarding Saxagliptin Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Saxagliptin in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Saxagliptin in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Saxagliptin in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Saxagliptin in the drug label.
How Supplied
Storage
There is limited information regarding Saxagliptin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Saxagliptin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Saxagliptin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Saxagliptin in the drug label.
Precautions with Alcohol
- Alcohol-Saxagliptin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Saxagliptin |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Saxagliptin |Label Name=Saxagliptin11.png
}}
{{#subobject:
|Label Page=Saxagliptin |Label Name=Saxagliptin11.png
}}