Sandbox:Cherry
- One fifth of the Americans with CAD have CTO (chronic total occlusion).
- CTO lesions are commonly ischemic, and studies have shown that despite collateral circulation, normal coronary flow reserve is found in less than 10% of patients. The presence of CTO has been associated with worse cardiovascular outcomes and death in select patient populations.
- Despite the presence of symptoms and objective evidence of ischemia or viability in the CTO myocardial territory, revascularization rates for such patients remain low.
- Here we will briefly review the improvements in current techniques, cardiovascular outcomes associated with successful CTO percutaneous coronary intervention (PCI), and discuss the current guidelines and appropriate use criteria for CTO PCI in stable ischemic heart disease (SIHD).
Current Progress
Historically, CTO PCI attempt rates have been low and vary considerably among centers. In fact, the presence of a CTO is one of the most common reasons for referral for coronary artery bypass grafting. Similarly, those patients who are not candidates for surgery, or choose not to have surgery, are frequently not offered percutaneous revascularization and treated with only medical therapy. CTO findings in the SYNTAX (Synergy Between Percutaneous Coronary Intervention With TAXUS and Cardiac Surgery) trial was the most common reason for incomplete revascularization. The low rates of CTO PCI stem from the historic low success rates and potential for increased complications. Over the past decade, however, there has been a significant improvement in the technologies available for CTO PCI as well as the international dissemination of advanced CTO PCI techniques and skill sets. Technological advancements have included the development of specific CTO wires including soft tip wires to facilitate retrograde wiring, penetration wires to facilitate puncture of the proximal and distal caps as well as to facilitate re-entry from the subintimal space. Dedicated microcatheters for collateral channel dilatation as well as dedicated re-entry systems have been similarly developed to facilitate the success of both retrograde and dissection and re-entry techniques.7-8 The most important measure to improve success rates of CTO PCI came from the development of the hybrid algorithm for CTO PCI, which created a procedure plan for each CTO based on four anatomic variables:
The ambiguity of the proximal occlusion cap The quality of the distal coronary segment after the CTO The operator ability to cross the collaterals The length of the occlusion segment9 The hybrid algorithm for CTO PCI combines all available crossing techniques (antegrade wire escalation, antegrade dissection and re-entry, and retrograde approaches) to optimize procedural efficacy, efficiency, and safety.9 The hybrid algorithm also emphasizes adopting the following three principles:
Clinical indication drives the decision for CTO PCI attempt. Lesion characteristics decide the initial procedure approach. The provision of an "exit failure mode" aims to increase procedure efficiency by decreasing the amount of time trying a single approach. The hybrid algorithm and the three principles culminated in the improved success rates, procedure efficiency, and safety of present day CTO PCI. The OPEN CTO (Outcomes, Patient Health Status, and Efficiency in Chronic Total Occlusion) study, the most contemporary CTO PCI registry available to date, reveals current trends in modern day CTO PCI, including a technical success rate of 86% (using core laboratory adjudication) with an associated risk of major adverse cardiac and cerebrovascular events (MACCE) of 7% and 0.9% risk of death.10
Cardiovascular Outcomes
Successful CTO PCI has been associated with numerous cardiovascular benefits, including improvement in left ventricular ejection fraction (LVEF), improved wall motion of the affected segment, and reduction in arrhythmic vulnerability.11-16 Quality-of-life variables have also been shown to improve after successful CTO PCI, including improvements in angina, heart failure symptoms, physical activity, and overall treatment satisfaction.10,17-18 Most importantly, successful CTO PCI has been associated with a reduction in all-cause mortality in registry data and met-analysis.19-20 Goa et al. published a meta-analysis of 5,958 patients who had undergone successful CTO PCI and compared them with 1,511 patients who had undergone unsuccessful CTO PCI.21 They found that successful CTO PCI using drug-eluting stents was associated with lower long-term mortality, lower risk of myocardial infarction, and lower risk of MACCE. Similarly, CTO PCI allows for complete revascularization, which has been associated with lower long-term mortality, lower rates of myocardial infarction, and lower rates of repeat revascularization, independent of the initial revascularization strategy. A post-hoc analysis of the 4-year outcomes of the SYNTAX trial showed the presence of CTO as the strongest predictor of incomplete revascularization after PCI, and incomplete revascularization was associated with higher adverse events, including increased mortality.22
There have been 3 randomized control trials to date evaluating CTO PCI outcomes. The EXPLORE (Evaluating Xience and Left Ventricular Function in PCI on Occlusions After STEMI) trial included 304 patients with ST-segment elevation myocardial infarction who had a non-culprit CTO diagnosed at the time of their ST-segment elevation myocardial infarctions.23 Patients were assigned to CTO PCI within 1 week of the initial event versus medical therapy. The investigator found that CTO PCI was feasible and safe; however, it did not provide any overall benefit in LVEF or left ventricular end diastolic volume. Interestingly, the subgroup of patients who underwent CTO PCI of the left anterior descending artery, who likely had the largest amount of myocardium at risk, did improve their LVEF (47.2 vs. 40.4%, p = 0.02). The DECISION-CTO (Optimal Medical Therapy With or Without Stenting For Coronary Chronic Total Occlusion) trial, which was terminated early due to slow enrollment, randomized 417 patients to optimal medical therapy and CTO PCI and 398 patients to optimal medical therapy alone. There were no differences in the primary outcome of mortality at 3 years or secondary outcomes, including repeat revascularization, mortality at 5 years, or quality of life. In addition to early termination, there were several limitations to the DECISION-CTO trial design, including the lack of assessment of symptoms once non-CTO lesions were revascularized and the lack of assessment for ischemia and viability in the myocardial territories supplied by the CTO. Interestingly, the per-protocol and as-treated analysis revealed a trend toward better outcome with CTO PCI versus optimal medical therapy. The trial has yet to be peer-reviewed and published. Lastly, EURO-CTO (A Randomized Multicentre Trial to Evaluate the Utilization of Revascularization or Optimal Medical Therapy for the Treatment of Chronic Total Occlusions), which was also terminated early secondary to slow enrollment, randomized 407 patients with CTO in a 2:1 fashion to either optimal medical therapy and CTO PCI or optimal medical therapy alone. The investigators found that patients who received CTO PCI had significant improvement in angina frequency when compared with optimal medical therapy (p = 0.009) and improvement in their Canadian Cardiovascular Society Angina scores (p < 0.001). There were similar MACCE rates at 12 months between the 2 arms.
Two out of the three randomized trials of CTO PCI were terminated early and did not meet their target enrollment numbers secondary to slow enrollment, which indicates the potential of significant selection bias. Patients who were enrolled are likely to have fewer symptoms and lower risk ischemia.
Clinical trials comparing optimal medical therapy and CTO PCI versus optimal medical therapy alone are therefore still needed. Investigators performing such trials face significant difficulties, including high cost to conduct randomized control trials, barriers to randomize patients who are highly symptomatic, and the relative few number of centers with high CTO PCI success rates to avoid bias against CTO PCI in an intention-to-treat analysis.
Guidelines
The 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention recommended CTO PCI in patients with clinical indications and suitable anatomy when performed by operators with appropriate expertise (Class IIa, Level of Evidence [LOE] B).24
The 2014 European Society of Cardiology and European Association for Cardio-Thoracic Surgery guidelines on myocardial revascularization recommend CTO PCI to be considered in patients with expected ischemia reduction in a corresponding myocardial territory and/or angina relief (Class IIa, LOE B). They recommend an initial anterograde approach and consideration of a retrograde approach if this fails or a primary retrograde approach in selected patients (Class IIb, LOE C).25
The ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease have eliminated the separate criteria for CTO lesions as was the case in the 2012 guidelines. Currently, indications for revascularization in SIHD are determined irrespective of whether the lesion is a CTO.26 The indication for revascularization of a coronary artery lesion, whether CTO or severe stenosis, is based on symptoms, the extent of antianginal medications, and the risk of ischemia.
Conclusions
As adoption of CTO PCI becomes more common and as evidence of cardiovascular benefits continues to evolve, guidelines have favorably acknowledged the appropriateness of CTO PCI. Currently, due to the lack of evidence from randomized control trials, routine use of CTO PCI is not recommended. Patients who have symptoms of ischemia despite optimal medical therapy, those with moderate to large areas of myocardium at jeopardy, and patients with ischemic cardiomyopathies with viable myocardium are likely to gain the most benefit from CTO PCI. CTO PCI should be performed by operators who are well versed in CTO PCI techniques and management of associated complications. Most importantly, informed discussions with patients and providers emphasizing the risk and benefits associated with CTO PCI for a given patient's condition are the keys to providing patients with appropriate CTO PCI.
6 Coronary Revascularization in Patients With Stable Ischemic Heart Disease: Appropriate Use Criteria (By Indication) Section 1. SIHD Without Prior CABG The format for tables in Section 1 is similar, with separate tables for 1-, 2-, and 3-vessel disease and left main disease. The columns in each table are stratified into 2 categories. There is a single column combining patients who are asymptomatic and not receiving antianginal therapy with patients who are asymptomatic and receiving antianginal therapy. The remaining columns are devoted to patients with ischemic symptoms, with 3 separate categories: ischemic symptoms and receiving no antianginal therapy, ischemic symptoms and receiving 1 antianginal drug (beta blocker preferred), and ischemic symptoms receiving 2 or more antianginal drugs. As outlined in the SIHD guideline, in the absence of contraindications, initial therapy should be a beta blocker prescribed at a dose that reduces heart rate without excessive resting bradycardia, hypotension, or fatigue. Other antianginal drugs are then added to beta blockers depending on the individual needs of the patient until symptoms are suppressed to the satisfaction of the patient or higher doses cannot be used because of side effects. In each of the subordinate columns, the panel was asked to rate the options for revascularization, specifically PCI or CABG. As noted earlier, the rating panel was asked to rate each revascularization option independent of the other, with the intent to rate each therapy on its own merits rather than in comparison to the other option. In this construct, both revascularization options could be assigned identical ratings.
In this and subsequent tables, clinical scenarios often contain the phrase “noninvasive testing.” In this document, that phrase includes all forms of stress testing using either dynamic or pharmacological stress that may be coupled with various imaging tests. It also could include other imaging, such as coronary computed tomography angiography or magnetic resonance imaging, to assess myocardial viability. Some would favor the term “functional testing,” but the writing committee did not view this as inclusive of computed tomography or magnetic resonance imaging and thus favored the term “noninvasive testing.” FFR is considered as part of an invasive evaluation and is cited separately in some scenarios. An emerging technology, computed tomography-derived FFR is a combination technique that is noninvasive like computed tomography but provides FFR, which has traditionally only been an invasive test.
Table 1.1. One-Vessel Disease VIEW INLINE VIEW POPUP Table 1.1 One-Vessel Disease
Similar to the 2011 CABG and 2012 SIHD guidelines, this document uses proximal LAD disease as an additional anatomic discriminator for 1-vessel CAD. Although data are minimal, the writing committee felt that proximal disease of a dominant circumflex should be considered as high-risk anatomy with similar implications as proximal LAD disease, and thus, it was considered in a separate section along with proximal LAD disease.
Table 1.2. Two-Vessel Disease VIEW INLINE VIEW POPUP Table 1.2 Two-Vessel Disease
The format of this table is similar to that for 1-vessel disease. Similar to the 2011 CABG and 2012 SIHD guidelines, this document makes a distinction regarding the presence or absence of proximal LAD disease. The writing group did not add proximal left dominant circumflex disease as an additional discriminator, because most would consider an isolated stenosis in this location to be the equivalent of 2-vessel disease (i.e., right coronary artery and circumflex disease). Following this construct, the combination of proximal LAD disease and proximal left dominant circumflex disease would be considered as 3-vessel disease and rated using the 3-vessel disease table (Table 1.3.). In the absence of exercise data, invasive physiological testing of both involved vessels is included in several of the indications. To remain in this table of 2-vessel disease, such testing must be abnormal in both vessels. If this testing shows only 1 vessel to be abnormal, the patient would no longer be rated using this table, but rather would be rated in the table for 1-vessel CAD. Finally, because of the increasing body of literature that has identified diabetes as an important factor to consider when recommending revascularization, scenarios indicating the presence of diabetes are provided.
VIEW INLINE VIEW POPUP Table 1.3 Three-Vessel Disease
Table 1.3. Three-Vessel Disease Similar to Table 1.2., because of the increasing body of literature that has identified diabetes as an important factor to consider when recommending revascularization, categories indicating the presence or absence of diabetes are provided among the individual indications. Stenosis complexity is also an important factor to consider in any revascularization procedure, probably more so for PCI than for CABG. The SYNTAX (Synergy between Percutaneous Coronary Intervention with TAXUS and Cardiac Surgery) trial provided a comprehensive comparison of PCI and CABG and a structure that may be helpful in formulating revascularization recommendations (35). Factors such as vessel occlusion, bifurcation or trifurcation at branch points, ostial stenosis location, length >20 mm, tortuosity, calcification, and thrombus all add to the complexity of PCI. The presence of multiple complex features (SYNTAX score >22) is associated with more favorable outcomes with CABG. Although limitations of the SYNTAX score for certain revascularization recommendations are recognized and it may be impractical to apply this scoring system to all patients with multivessel disease, it is a reasonable surrogate for the extent and complexity of CAD and provides important information that can be helpful when making revascularization decisions.
Accordingly, in this table specifically for patients with 3-vessel disease, the rating panel was asked to consider the indications in patients with low complexity compared with those with intermediate and high complexity.
Table 1.4. Left Main Coronary Artery Stenosis VIEW INLINE VIEW POPUP Table 1.4 Left Main Coronary Artery Stenosis
Literature on the treatment of significant left main disease is dominated by surgical revascularization procedures and, more recently, comparisons with PCI in some anatomic situations. There are data suggesting that stenting of the left main ostium or trunk is more straightforward than treating distal bifurcation or trifurcation stenoses and is associated with a lower rate of restenosis. In comparison, left main lesion location has a negligible influence on the success and long-term results of CABG. Accordingly, there are separate rating options for ostial and mid-shaft left main disease and distal or bifurcation left main disease. The definition of a significant left main stenosis used herein is ≥50% narrowing by angiography. However, the angiographic assessment of the severity of left main disease has several shortcomings, and other assessments such as IVUS or FFR may be needed. For left main coronary artery stenoses, a minimum lumen diameter of <2.8 mm or a minimum lumen area of <6 mm2 suggests a physiologically significant lesion. It has been suggested that a minimum lumen area >7.5 mm2 suggests revascularization may be safely deferred. A minimum lumen area between 6 and 7.5 mm2 requires further physiological assessment, such as measurement of FFR. Alternatively, FFR may be used as the first modality to assess ambiguous left main severity, and the criteria for a significant stenosis are the same as for non–left main stenosis (21,36,37).
Section 2. Tables 2.1 and 2.2 SIHD With Prior CABG VIEW INLINE VIEW POPUP Table 2.1 IMA to LAD Patent and Without Significant Stenoses
VIEW INLINE VIEW POPUP Table 2.2 IMA to LAD Not Patent
Patients with prior CABG surgery can present with a wide spectrum of disease progression. This includes the development of new obstructive disease in coronary arteries not bypassed in the first operation, new stenoses in existing bypass grafts, and territory previously bypassed but jeopardized again because of graft occlusion. Developing indications inclusive of all of these anatomic possibilities would be impractical. Accordingly, the writing committee adopted a more compact construct based on the presence of a significant stenosis in a bypass graft or native coronary artery supplying 1, 2, or 3 distinct vascular territories roughly corresponding to the territories of the 3 main coronary arteries. As in patients without prior CABG, the indications included an assessment of risk based on noninvasive testing (low versus intermediate or high risk).
Evaluation of the severity and physiological significance of a stenosis in saphenous vein grafts (SVG) can be particularly challenging because of the usual marked size difference between the SVG and native artery. Although FFR measurements are well-validated in native vessels, data on the use of FFR in vein grafts are limited (38). After CABG surgery, the bypass conduit should act in a similar fashion to the native, low-resistance epicardial vessel. However, the assessment of ischemia due to a stenosis in a vein graft is complicated by several features, which include: 1) the potential for competing flow (and pressure) from both the native and conduit vessels; 2) the presence of collaterals from longstanding native coronary occlusion; and 3) the potential for microvascular abnormalities due to ischemic fibrosis and scarring, pre-existing or bypass surgery–related myocardial infarction, or chronic low-flow ischemia. Despite these complicating features, the theory of FFR should apply equally to both a lesion in an SVG to the right coronary artery feeding a normal myocardial bed and a lesion in the native right coronary. However, if the native and collateral supply are sufficiently large, the FFR across an SVG stenosis could be normal. FFR measurements may be most useful in the setting of an occluded bypass graft to a native artery with an intermediate-severity stenosis. FFR measurements in bypass grafts are less well-validated and should thus be interpreted with caution.
Two tables are presented for the rating of patients with prior CABG depending on the patency of an existing internal mammary artery (IMA) graft. IMAs have a greater long-term patency rate than SVGs—typically >90% after 10 years (39,40). Accordingly, use of the IMA as a conduit in CABG surgery has steadily increased. Current use is 98%, as reported in the Society of Thoracic Surgeons national database, and use of the IMA as a conduit is 1 of the quality metrics in their composite score. Because of the current high use of the IMA, the writing committee felt there were too few patients to consider a separate category consisting of patients who only had SVGs used in their first operation, although a few such patients may exist. Moreover, the writing committee did not develop any scenarios where the initial operation consisted of only bypass grafts to the circumflex and right coronary artery in the absence of LAD disease. The patency and longevity of the IMA as a bypass graft was felt by the writing committee to be an important decision point in the indication development, as many cardiovascular surgeons are hesitant to perform a second bypass operation in the presence of a patent and fully functional IMA graft, especially to the LAD. The path of the IMA, particularly if it courses medially or is adherent to the back of the sternum, may be at greater risk during sternal re-entry, with adverse consequences even if the IMA-grafted vessel is regrafted. For Table 2.1., it is assumed that the LAD was significantly diseased at the time of the original operation. Therefore, if the IMA to the LAD is no longer patent or is severely diseased, it is assumed that the native LAD is also severely diseased or occluded.
Section 3. Table 3.1 SIHD Undergoing Procedures for Which Coronary Revascularization May Be Considered VIEW INLINE VIEW POPUP Table 3.1 Stable Ischemic Heart Disease Undergoing Procedures for Which Coronary Revascularization May Be Considered
In an effort to capture common clinical scenarios that are not well-represented in guidelines, the writing group developed indications for preoperative revascularization in patients being evaluated for renal transplantation or structural heart procedures. The writing committee recognized that pre-operative revascularization is sometimes requested before transplantation of other organs, but there is insufficient experience or data from controlled studies upon which to develop meaningful scenarios. These scenarios do not capture all possible clinical situations, but were felt to capture the majority of common clinical situations. If patients have an acute coronary syndrome, the writing group felt they should be rated according to the AUC for acute coronary syndrome. For many of these patients, symptoms may be difficult to attribute to myocardial ischemia; thus, the indications used in this table provide only anatomic and noninvasive test findings for review. Note that for patients being evaluated before a percutaneous valve procedure, the option for CABG surgery is blocked out, as it is assumed such patients have clinical factors making their risk of surgery prohibitively high.
7 Discussion The AUC are intended to inform clinicians, patients, and health policy makers about the reasonable use of technologies to help improve patient symptoms and health outcomes. Since 2005, the American College of Cardiology, along with its professional partners, has worked to provide criteria for both invasive and noninvasive testing and selected treatments, with the intention of further expanding the AUC portfolio.
The 2017 Appropriate Use Criteria for Revascularization in Patients With Stable Ischemic Heart Disease is the culmination of approximately 2 years of review and revision to the existing AUC. In response to comments from multiple stakeholders, the current AUC has several important changes (41). First, this document will use the new terms “appropriate care,” “may be appropriate care,” and “rarely appropriate care,” which were described in the updated AUC methodology paper (2). Second, the composition of the rating panel was changed slightly to include 5 cardiac surgeons, 5 interventional cardiologists, 6 cardiologists not directly involved with performing revascularization, and 1 outcomes researcher. Third, the new criteria stratify symptoms into 2 general groups—asymptomatic and ischemic symptoms—to be inclusive of the spectrum of complaints that may occur from myocardial ischemia. Furthermore, because of the variety of symptoms that may indicate myocardial ischemia, individual patient variation in how they are described, and observer variability in the assessment of symptom severity, the writing group chose to abandon the Canadian Cardiac Society classification. However, the current criteria continue to emphasize the use of more objective measures of ischemia within indications to stratify patients into low-risk or intermediate-/high-risk findings, as described in the SIHD guideline. Fourth, the scenarios expand the use of intracoronary physiological testing, mainly with FFR. Fifth, the structure of the AUC tables concerning the use of antianginal therapy has changed to reflect typical practice patterns rating patients on the basis of no antianginal therapy, use of 1 antianginal drug, or use of 2 or more antianginal drugs. As in earlier documents, it is assumed that all patients are being treated with guideline-directed medical therapies to reduce risk. Finally, in an effort to capture patients who have not previously been categorized, the current AUC also rate coronary revascularization in patients being considered for renal transplantation and percutaneous valve procedures.
Review of the ratings demonstrate some themes around revascularization of patients with SIHD that are consistent with existing clinical practice guidelines. In general, in patients with a low burden of coronary disease (e.g., single-vessel disease), low-risk findings on noninvasive testing, and/or no antianginal therapy, revascularization by PCI or CABG surgery for care is felt to be rarely appropriate as the initial step. As disease burden progresses through 2-vessel to 3-vessel and left main disease, revascularization by PCI or CABG frequently becomes rated as “may be appropriate care” or “appropriate care,” with CABG surgery consistently rated as “appropriate care” for intermediate or high disease complexity (SYNTAX ≥22) even in patients with ischemic symptoms who are not on antianginal therapy. Of note, CABG surgery was consistently rated as “appropriate care” and PCI as “rarely appropriate care” for left main bifurcation disease with intermediate or high disease burden in other vessels.
Repeat CABG surgery was felt to be rarely appropriate in patients with a functional patent IMA to the LAD in all but 1 indication, with both PCI and CABG being rated as either “may be appropriate care” or “appropriate care” in the other indications, reflecting the complex and individualized decision making required in these patients. With the exception of a few specific scenarios in asymptomatic patients with a low disease burden, revascularization options were considered as “may be appropriate care” or “appropriate care” options. Although not directly rated, the use of fractional flow reserve for evaluation of renal transplant patients may be considered and will be addressed in future revascularization documents. Revascularization by PCI was considered appropriate care for the majority of patients being evaluated before a percutaneous valve procedure.
Application of Criteria There are many potential applications for AUC, including their adoption by Centers for Medicare & Medicaid Services regulators as a means of evaluating care. Clinicians can use the ratings for decision support or as an educational tool when considering the need for revascularization. Moreover, these criteria can be used to facilitate discussions with patients and/or referring physicians about the need for revascularization. The original intent of the AUC was to provide a tool to identify patterns of care, including both the overuse and underuse of various services. In fact, some of the initial publications related to AUC identified underuse and the consequences of underuse rather than overuse of services (42,43). Facilities have used these criteria to design protocols to facilitate the appropriate care of patients. Some payers have adopted the AUC for use in the preauthorization of procedures or retrospectively for quality reports. Although the AUC were never intended to determine payment in individual patients, some payers have adopted the AUC for this purpose. The desire of payers to control costs is understood, but it should be in the context of developing rational payment management strategies to ensure their members receive necessary, beneficial, and cost-effective cardiovascular care, rather than for other purposes. It is expected that services performed for “appropriate” or “may be appropriate” indications will receive reimbursement. In contrast, services performed for “rarely appropriate” indications should be justified by additional documentation to justify payment because of the unique circumstances or the clinical profile that must exist in such a patient. It is critical to emphasize that the writing group, technical panel, Appropriate Use Criteria Task Force, and clinical community do not believe a rating of “may be appropriate” is justification to deny reimbursement for revascularization. Rather, “may be appropriate” ratings are those in which the available data vary and many other factors exist that may affect the decision to perform or not perform revascularization. The opinions of the technical panel often varied for these indications, reflecting that additional research is needed.
The writing group recognizes the need to align the collection of clinical data required for the determination of appropriate use with appropriate methods to reduce the burden of data collection. To this end, the NCDR CathPCI Registry group has been engaged in a parallel process to ensure that needed data elements are incorporated into the Registry. The criteria will also be evaluated for collection by the Society for Thoracic Surgeons registry. Incorporating fields to identify patients who are not felt to be candidates for PCI or CABG surgery has been suggested to ensure proper mapping of the AUC in the course of medical decision making. The writing committee believes the key step to ensuring that the AUC are iterated and continually improved is the use of a feedback cycle of data between current clinical practice and the Registry. The writing group also believes that the mapping of the data elements on the NCDR CathPCI Registry data collection from the AUC should be transparent for all providers to review and implement local systems of care.
In conclusion, this document represents the current understanding of the clinical benefit of coronary revascularization with respect to health outcomes and survival. These criteria have been developed through the AUC process and alignment with the evidence and recommendations from clinical practice guidelines. This is intended to provide a practical guide to clinicians and patients when considering revascularization. As with all AUC, some of these ratings will require research and further evaluation to provide the greatest information and benefit to clinical decision making. We anticipate that the utility and ability of these criteria to improve the quality of care will be measured by the overall use and adoption of the criteria. With each update, the AUC for coronary revascularization in SIHD have become more refined and specific, while areas for continued focus and research have been identified.
Physical examination
References
References
Pathophysiology prev
| https://https://www.youtube.com/watch?v=5szNmKtyBW4%7C350}} |
|
Cirrhosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case studies |
|
Sandbox:Cherry On the Web |
|
American Roentgen Ray Society Images of Sandbox:Cherry |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief:
Pathophysiology prev
| https://https://www.youtube.com/watch?v=5szNmKtyBW4%7C350}} |
|
Cirrhosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case studies |
|
Sandbox:Cherry On the Web |
|
American Roentgen Ray Society Images of Sandbox:Cherry |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2] Associate Editor(s)-in-Chief:
History and Symptoms
- History should include:
- Appearance of bowel movements
- Travel history
- Associated symptoms
- Immune status
- Woodland exposure
References
Other Imaging Findings
Other diagnostic studies
Other Diagnostic Studies
- Breath hydrogen test
- HIV testing for those patients suspected of having HIV
==
Overview
References
Pathophysiology prev
| https://https://www.youtube.com/watch?v=5szNmKtyBW4%7C350}} |
|
Cirrhosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case studies |
|
Sandbox:Cherry On the Web |
|
American Roentgen Ray Society Images of Sandbox:Cherry |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3] Associate Editor(s)-in-Chief:
Video codes
Normal video
{{#ev:youtube|x6e9Pk6inYI}} {{#ev:youtube|4uSSvD1BAHg}} {{#ev:youtube|PQXb5D-5UZw}} {{#ev:youtube|UVJYQlUm2A8}}
Video in table
Floating video
| Title |
| https://https://www.youtube.com/watch?v=ypYI_lmLD7g%7C350}} |
Redirect
- REDIRECTEsophageal web
synonym website
https://mq.b2i.sg/snow-owl/#!terminology/snomed/10743008
Image
Image to the right
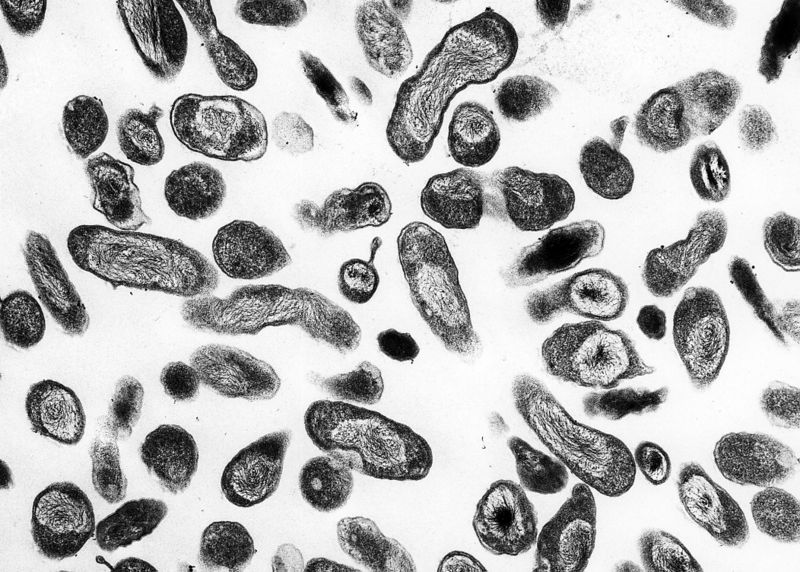 |
Image and text to the right
<figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline><figure-inline>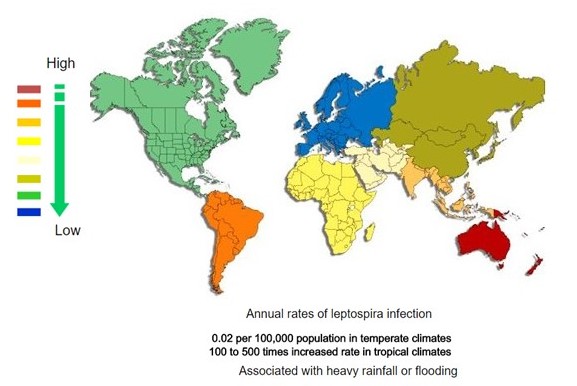 </figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline> Recent out break of leptospirosis is reported in Bronx, New York and found 3 cases in the months January and February, 2017.
</figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline></figure-inline> Recent out break of leptospirosis is reported in Bronx, New York and found 3 cases in the months January and February, 2017.
Gallery
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[1]
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[1]
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Insulin immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[1]
References
- ↑ 1.0 1.1 1.2 Neuroendocrine tumor of the pancreas. Libre Pathology. http://librepathology.org/wiki/index.php/Neuroendocrine_tumour_of_the_pancreas
REFERENCES
![Histopathology of a pancreatic endocrine tumor (insulinoma). Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[1]](/images/2/2f/Pancreatic_insulinoma_histology_2.JPG)
![Histopathology of a pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[1]](/images/a/a3/Pancreatic_insulinoma_histopathology_3.JPG)
![Histopathology of a pancreatic endocrine tumor (insulinoma). Insulin immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[1]](/images/d/d5/Pancreatic_insulinoma_histology_4.JPG)