Sandbox:Aditya
Causes
Common causes
- Peptic ulcer disease
- Responsible for around 33%-50% of upper GI bleeding
- Peptic ulcer disease is most commonly due to H.pylori or nonsteroidal anti-inflammatory drugs (NSAIDs).
- Upper GI bleeding is the most common complication of peptic ulcer disease and may be the initial presentation.[1]
- Esophageal varices
- Mallory-Weiss syndrome :
- Responsible for around 5% of upper GI bleeding
- A longitudinal mucosal laceration in the distal esophagus and/or proximal stomach that usually results from forceful retching
Less common causes
- Neoplasms
- gastric cancer
- esophageal tumors
- Esophagitis (complications due to erosive or necrotizing infectious esophagitis )
- Gastric erosions/gastropathy [4]
- Dieulafoy lesions
- Dilated aberrant submucosal vessels that erode the overlying epithelium in the absence of an ulcer
- Gastric varices
- Gastric antral vascular ectasia
- Dilated gastric vessels of unknown etiology that cause chronic UGIB and iron-deficiency anemia
Rare causes
- Bleeding from the hepatobiliary tract
- Most commonly secondary to a liver or biliary tract injury, from trauma or following procedures or surgery.
- Diagnosed by endoscopic retrograde cholangiopancreatography (ERCP) and treated with arteriography
- Aortoenteric fistulas,
- Most commonly involves the lower duodenum.
- Common causes include aortic aneurysms or prosthetic vascular grafts, syphilis and tuberculosis
- Presents with frank UGIB along with a pulsatile mass and abdominal pain radiating to the back.
- Diagnosed by endoscopy.
- Crohn disease involving the upper gastrointestinal tract
- Metastatic malignancy involving the upper gastrointestinal tract, such as melanoma or renal cell carcinoma
- Hemosuccus pancreaticus
- Pancreatic inflammation or cancer may result in bleeding into the pancreatic duct, which connects to the duodenum
Risk factors
- Advancing age[7][8][9][10]
- Previous history of gastrointestinal bleed
- Chronic kidney disease
- Underlying cardiovascular disease
- Cirrhosis and portal hypertension
- Presence of H.pylori
- NSAID or aspirin use in patients with a history of ulcer disease
- Those on dual antiplatelet therapy; those on anticoagulant therapy; or those with two or more of the following risk factors
- Age 60 years or older
- Glucocorticoid use
- Dyspepsia
- Gastroesophageal reflux disease
- Those on dual antiplatelet therapy; those on anticoagulant therapy; or those with two or more of the following risk factors
- Critical illness
- Nosocomial stress ulcers due the to the use of mechanical ventilation for more than 48 hours, and coagulopathy
- Other risk factors for nosocomial stress ulcerations in critically ill patients include a history of gastrointestinal ulceration or bleeding within the past year; or two or more of the following risk factors: presence of sepsis, ICU admission lasting longer than 1 week, occult gastrointestinal bleeding lasting 6 days or longer, and administration of more than 250 mg of hydrocortisone or equivalent glucocorticoid therapy
- Rare conditions associated with gastric acid hypersecretion, such as Zollinger-Ellison syndrome, mastocytosis, or a retained antrum following partial gastrectomy.
| Causes of Acute Upper GI bleeding | |
|---|---|
| Esophagus |
|
| Gastric |
|
| Duodenal |
|
Associated Conditions
- Heyde syndrome, aortic valve stenosis with associated gastrointestinal bleeding thought to be due to acquired reduction of von Willebrand factor.[11]
History
Obtaining the history is the most important aspect of making a diagnosis of upper GI bleed. It provides insight into the cause, precipitating factors and associated comorbid conditions and also helps in determining the severity of the bleed as well as in identifying the potential source of bleed:[12][13]
- A history of epigastric pain, dyspepsia, or prior peptic ulcer may suggest the diagnosis of peptic ulcer disease.
- A history of documented prior upper GI bleeding is important because approximately 60% of upper GI bleeders are rebleeding from the same site.
- Prior use of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs) is important because these patients have an increased risk of gastric ulcer and a fourfold risk of significant GI bleeding compared with other patients.
- A history of alcoholism increases the likelihood of cirrhosis and consequently of bleeding from esophageal varices or congestive gastropathy but alcoholics also frequently have peptic ulcers or gastritis.
- Cigarette smokers have a significantly higher rate of the recurrent duodenal ulcer as compared with nonsmokers and a history of cigarette smoking should be elicited.
- Vomiting, coughing, or retching before bleeding is suggestive of a Mallory-Weiss tear.[14]
A directed history may also alert to consider unusual causes.[15]
- A history of pancreatitis suggests possible hemorrhage from a pancreatic pseudocyst. Erosion of a pancreatic pseudocyst into the duodenum or stomach may cause massive hematemesis, and the patient may present in shock.
- Patients with prior abdominal aortic aneurysm repair may present with severe GI hemorrhage from an aortoenteric. This fistula often presents with a herald bleed followed within 4 to 96 hours by massive bleeding.
- A personal or family history of recurrent epistaxis may suggest the diagnosis of Osler-Weber-Rendu syndrome (hereditary hemorrhagic telangiectasia), and a careful examination for skin telangiectasias should be performed.
- Patients with renal failure frequently have GI bleeding. This bleeding is often due to peptic ulcer disease or angiodysplasia. This bleeding may be severe because of clotting dysfunction associated with renal disease.
Primary Prevention
Effective measures for the primary prevention of upper GI bleeding include administration of PPI in patients with an increased risk due to critical illness or use of NSAIDs or aspirin. In patients with cirrhosis and suspected portal hypertension, who found to have esophageal varices patients are given prophylactic treatment with a nonselective β-blocker or undergo endoscopic variceal ligation (EVL) with surveillance endoscopy.
Patients with stress ulcers
- The American Society of Health-System Pharmacists developed clinical practice guidelines that recommend prophylaxis with a PPI or with a histamine-2 receptor antagonist (H2RA) for ICU patients at high risk for UGIB.[18][19][20]
Patients on NSAID, aspirin, or antiplatelet therapy
- Joint gastroenterology and cardiology society practice guidelines recommend gastroprotective therapy with a PPI for patients considered to be at increased risk of bleeding from chronic NSAID and aspirin therapy.
Patients with cirrhosis and varices
- EGD is used to screen for the presence of varices in patients with cirrhosis complicated by portal hypertension.
- In patients with cirrhosis who do not have varices, no prophylaxis is indicated.
- In patients with cirrhosis and varices that have not bled, prophylactic treatment with nonselective β-blockers, such as nadolol or propranolol, may decrease portal blood flow and thus decrease the risk of variceal bleed.
- In patients with cirrhosis who have medium or large varices that have not bled, EVL is an alternative prophylactic treatment.
- EVL is repeated every several weeks until obliteration of varices is seen.
- Surveillance EGD should then be performed 1 to 3 months after obliteration and then every 6 to 12 months to check for variceal recurrence.
Secondary Prevention
Effective measures for the secondary prevention of UGIB include discouraging the use of NSAIDS in all patients with a history of UGIB.
Seondary Prevention
- NSAID use in all patients with a history of UGIB should be discouraged.[21]
UGIB from peptic ulcer disease
- Avoid NSAIDs.
- For patients who are at high risk for rebleeding (elderly patients; those taking anticoagulant and antiplatelet medications), indefinite use of a PPI may be recommended.[22]
- H pylori status should be determined, and patients should be treated if positive.
- Eradication is confirmed with stool sample or repeat endoscopy with biopsy.
UGIB from varices
- A combination of nonselective β-blockers plus EVL is the best option for secondary prophylaxis of UGIB from varices.
- The nonselective β-blocker should be titrated up as tolerated.
- Variceal banding should be repeated every 2 to 3 weeks until the varices are obliterated.
- EGD must be performed 1 to 3 months after initial obliteration then every 6 to 12 months to check for variceal recurrence.
Prognosis
- Prognosis is generally good with appropriate treatment, and the 1-year mortality rate of patients with nonvariceal UGIB is approximately 10%.[23][24][25][26]
- In UGIB, the prognosis doesn't depend on the severity of bleeding but depends upon patients age and comorbid conditions.
- The majority of patients with UGIB will stop bleeding spontaneously.
- A clean ulcer base has less than a 3% chance of rebleeding; therefore, these lesions are not usually treated or scoped again.
- In otherwise stable patients, patients with a clean ulcer base has less than a 3% chance of rebleeding and are good candidates for early discharge.
- Treatment includes management of underlying liver disease and prevention of complications of cirrhosis.
- Despite advances in gastric acid suppression as well as improved endoscopic diagnostic and therapeutic techniques, the mortality rate from UGIB has remained stable.
Scoring systems
Two scoring systems identify those at risk for adverse outcomes from UGIB:[27]
- The Glasgow Blatchford Score (GBS)
- The Rockall score
The Glasgow Blatchford Score (GBS)
- The Glasgow Blatchford Score (GBS) helps in identifying low-risk patients with UGIB who can be managed safely as outpatients without an urgent endoscopy.[28][29]
- GBS parameters include
- Blood urea nitrogen level
- Hematocrit level
- Heart rate
- Systolic blood pressure
- Presence of syncope or melena, as well as presence of comorbid heart and liver disease.
- GBS is the more effective system for predicting the need for transfusion in patients with UGIB.
| The Glasgow Blatchford Score (GBS) | |||
|---|---|---|---|
| Admission risk markers | Score | ||
| Blood urea nitrogen level (mg/dl) | ≥ 18.2 to < 22.4 | 2 | |
| ≥ 22.4 to < 28 | 3 | ||
| ≥ 28 to < 70 | 4 | ||
| ≥ 70 | 6 | ||
| Hemoglobin level (g/dl) | Men | ≥ 12 to < 13 | 1 |
| ≥ 10 to < 12 | 3 | ||
| < 10 | 6 | ||
| Women | ≥ 10 to < 12 | 1 | |
| < 10 | 6 | ||
| Systolic blood pressure (mmHg) | ≥ 100 to < 109 | 1 | |
| ≥ 90 to < 99 | 2 | ||
| < 90 | 3 | ||
| Other markers | Pulse rate ≥ 100 beats/min | 1 | |
| Presentation with melena | 1 | ||
| Presentation with syncope | 2 | ||
| Hepatic disease | 2 | ||
| Heart failure | 2 | ||
|
Scores of 0-2 -Low-risk group | |||
The Rockall score
- The complete Rockall score identifies those patients with evidence of acute UGIB on endoscopy who are at low risk for further bleeding or death.[30][31]
- The score is based upon
- Age
- Presence of shock
- Comorbidity diagnosis
- Endoscopic ulcer characteristics
- Stigmata of recent hemorrhage.
| The Rockall score | |||
|---|---|---|---|
| Markers | Score | ||
| Age | <60 | 0 | |
| 60 - 79 | 1 | ||
| ≥ 80 | 2 | ||
| Shock stage | Blood pressure | >120 | 0 |
| 100-119 | 1 | ||
| <100 | 2 | ||
| Heart rate | >100 | 0 | |
| <100 | 1 | ||
| Comorbidity | No major comorbidity | 0 | |
| Cardiac failure
Ischemic heart disease Any major comorbidity |
2 | ||
| Renal failure
Liver failure Disseminated malignancy |
3 | ||
| Diagnosis | Mallory-Weiss tear, no lesion identified and no SRH | 0 | |
| All other diagnosis | 1 | ||
| Malignancy of upper GI tract | 2 | ||
| Major SRH | None or dark spot only | 0 | |
| Blood in upper GI tract, adherent clot, visible or spurting vessel |
2 | ||
| GI: Gastrointestinal, SRH: Signs of recent hemorrhage.
Range of score is 0-11. Score of ≤ 3 predicts low mortality risk, while ≥ 8 is a predictor of high mortality risk. | |||
Complications
Complications of UGIB include:[32]
- End-organ damage
- Cardiac ischemia
- Renal failure
- Ischemic hepatitis
- Anoxic brain injury
- Iron-deficiency anemia
Classification
According to The American Gastroenterological Association, upper GI bleeding can be classified based on the rate of blood loss into overt(acute), occult or obscure(chronic) forms.[33][13][34][35]
- Overt GI bleeding:- Overt GI bleeding is defined as acute bleeding which is visible and can present in the form of hematemesis, “coffee-ground” emesis, melena, or hematochezia.
- Occult or chronic GI bleeding:- Occult GI bleeding is defined as a microscopic hemorrhage which can present as Hemoccult-positive stools with or without iron deficiency anemia. It is the initial presentation in patients with no evidence of visible blood loos and is positive on fecal occult blood test(FOBT).
- Obscure GI bleeding:- Obscure GI bleeding is defined as recurrent bleeding in which a source is not identified after upper endoscopy and colonoscopy. It can be either overt or occult.
- Overt GI bleeding:- Overt GI bleeding is defined as acute bleeding which is visible and can present in the form of hematemesis, “coffee-ground” emesis, melena, or hematochezia.
Epidemiology and Demographics
Incidence
The incidence of acute UGIB is approximately 50 to 100 per 100,000 individuals worldwide.[36][6]
Gender
Males are more commonly affected by UGIB than females. The males to female ratio is approximately 2 to 1.
Pathophysiology
Blood supply of Foregut
The digestive system is supplied by the celiac artery. The celiac artery is the first major branch from the abdominal aorta, and is the only major artery that nourishes the digestive organs.[37][38][39][40][41][42][43]
| Foregut | Blood supply | |
|---|---|---|
| Esophagus |
Upper esophageal sphincter |
Inferior thyroid artery |
| Thoracic esophagus | Aortic esophageal arteries or branches of the bronchial arteries | |
|
Distal esophagus |
Left gastric artery and left phrenic artery | |
| Stomach | Lesser curvature | Right and left gastric arteries |
| Greater curvature | Right and left gastroepiploic arteries | |
| Gastric fundus | Short gastric arteries | |
| Duodenum | First and second parts |
Gastroduodenal artery (GDA) and |
| Third and fourth parts | Inferior pancreaticoduodenal artery | |

Source: By Mikael Häggström.https://commons.wikimedia.org/w/index.php?curid=3416062
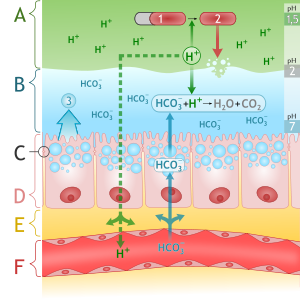
Mucosal barrier
- The gastric mucosa is protected from the acidic environment by mucus, bicarbonate, prostaglandins, and blood flow.[44][45][46]
- This mucosal barrier consists of three protective components which include:
- Layer of epithelial cell lining.
- Layer of mucus, secreted by surface epithelial cells and foveolar cells.
- Layer of bicarbonate ions, secreted by the surface epithelial cells.
Source: By M•Komorniczak (http://creativecommons.org/licenses/by/3.0)], via Wikimedia Commons
The following table demonstrates the defense mechanisms of gastric mucosal barrier[47]
| Defense mechanisms of gastric mucosal barrier | |
|---|---|
| Mucus layer | Forms a protective gel-like coating over the entire gastric mucosal surface |
| Epithelial layer | Epithelial cell layer are bound by tight junctions that repel fluids |
| Bicarbonate ions | Neutralize acids |
Pathogenesis
The main inciting event in the pathogeneis of upper GI bleeding is damage to mucosal injury. This mucosal injury can occur at various levels of GI tract. If the damage and bleeding is confined up to ligament of Treitz, it is defined as upper GI bleeding.[6][48]
| Etiology | Frequency of occurance |
|---|---|
| Peptic ulcer disease | 50% |
| Variceal bleeding | 20% |
| Esophagitis, gastritis, and duodenitis | 10-15% |
| Mallory-Weiss tear | 15% |
| Malignancy | 3-5% |
| Arteriovenous malformation | <3% |
| Gastric antral vascular ectasia | <1% |
| Dieulafoy lesion | <1% |
Pathogenesis
- Regardless of etiology, if the balance of gastric acid secretion and mucosal defenses is disrupted, acid interacts with the epithelium to cause damage.[49][50][51]
- Varices are large, tortuous veins and protrude into the lumen, rupturing.[52]
- Helicobacter pylori disrupts the mucosal barrier and causes inflammation of the mucosa of the stomach and duodenum.[53][54]
- As the ulcer progresses beyond the mucosa to the submucosa the inflammation causes weakening and necrosis of arterial walls, leading to pseudoaneurysm formation followed by rupture and hemorrhage.[55]
- NSAIDs inhibit cyclooxygenase, leading to impaired mucosal defenses by decreasing mucosal prostaglandin synthesis.[56]
- During stress, there is acid hypersecretion; therefore, the breakdown of mucosal defenses leads to injury of the mucosa and subsequent bleeding.
- Mucosal defects along with dilated and tortuous vessels in dieulafoy lesion put them at risk for rupture because of necrosis of the arterial wall from exposure to gastric acid.[57][58][59][60]
| NSAIDS | |||||||||||||||||||||||||||||||||||||||||||||||
| Inhibits cycloxygenase pathway | |||||||||||||||||||||||||||||||||||||||||||||||
| COX-1 | COX-2 | ||||||||||||||||||||||||||||||||||||||||||||||
| Reduced mucosal blood flow | Reduced mucosal and bicarbonate secreation | Impaired platelet aggregation | Reduced angiogenesis | Increased leucocyte adherence | |||||||||||||||||||||||||||||||||||||||||||
| Impaired defence Impaired healing | |||||||||||||||||||||||||||||||||||||||||||||||
| Mucosal Injury | |||||||||||||||||||||||||||||||||||||||||||||||
Gross and Microscopic Pathology
| Gross Pathology | Microscopic Pathology | ||
|---|---|---|---|
| Varices | Large and tortuous veins that protrude into the lumen | Varices may be difficult to demonstrate in surgical specimens | |
| Mallory-Weiss Tear[61] | Isolated or multiple cleft like mucosal defects |
| |
| Esophagitis[62] | Herpes esophagitis |
|
Ground glass inclusion bodies |
| Cytomegalovirus esophagitis |
|
Intranuclear inclusions | |
| Fungal esophagitis |
|
Neutrophils within the squamous epithelium | |
| Pill esophagitis |
|
Not specific and include
| |
| Toxic esophagitis |
|
Acid injury
Alkaline injury
| |
| Gastroesophageal
Reflux Disease[63] |
| ||
| Barrett Esophagus[64] | Columnar metaplasia
| ||
| Acute Gastritis | Mucosal hyperemia associated with
|
| |
| Gastric Ulcers[1] |
|
| |
| Portal Hypertensive Gastropathy[65] |
|
| |
| Gastric Antral Vascular Ectasia[65] | Linear pattern of mucosal congestion in the antrum termed “watermelon stomach | Antral biopsies show
The mucosa also shows
| |
| Reactive (Chemical) Gastropathy | Stomach
|
The mucosa shows
| |
| Peptic Disease | Wide range of findings
|
| |
| Ischemia | Hypoperfused ulcers | Acute ischemia
Chronic ischemia
| |
| Structural Abnormalities of Blood Vessels[66] | Large-caliber artery within the submucosa | Dilated venules and arteriole in direct communication with each other | |
| Inflammatory Bowel Disease | Lymphoplasmacytic infiltrate with numerous neutrophils | ||
Diagnosis
In patients with acute Upper GI bleeding who are unstable rapid assessment and resuscitation should be initiated even before diagnostic evaluation. Once hemodynamic stability is achieved, a proper clinical history, physical examination, and initial laboratory findings are crucial not only in determining the likely sources of bleeding but also in directing the appropriate intervention. The hematocrit level is measured soon after the onset of bleeding, but will not accurately reflect the amount of blood loss. Equilibration between the intravascular and extravascular spaces is not complete until 24 to 72 hours after bleeding has occurred. Low mean corpuscular volume, low iron and ferritin levels, and high transferrin and total iron-binding capacity (TIBC) confirm iron deficiency. Blood urea nitrogen (BUN) level may be elevated out of proportion to any increase in the creatinine level in patients with UGIB, secondary to the breakdown of blood proteins to urea by intestinal bacteria. Platelet count and coagulation studies should be checked, especially in patients with known or suspected coagulopathy. Nasogastric lavage should be performed if the presence or source of bleeding is unknown. Upper gastrointestinal endoscopy is the primary diagnostic tool, performed for both diagnosis and treatment of active bleeding. The American Society of Gastrointestinal Endoscopy guidelines recommend that upper endoscopy be performed within 24 hours of presentation in all patients with UGIB. Angiography and tagged erythrocyte scan are rarely needed, but may be used to diagnose (and embolize) active UGIB, particularly in patients who cannot tolerate EGD. Also, upper gastrointestinal tract radiographic studies using barium are generally not advised, as they may obscure visualization during EGD.[67][68][69][70]
Initial Laboratory Studies
- The hematocrit level is used to identify the degree of blood loss and suggests the acuity or chronicity of blood loss.[34][13]
- Serial complete blood count (CBC) tests are important for monitoring the presence of ongoing blood loss.
- Initial CBC may not fully reflect the actual degree of acute blood loss.
- Qualitatively, on peripheral blood smear prepared with Wright-Giemsa stain, normal erythrocytes should be smaller than the nucleus of a normal lymphocyte, and the central clear area should not be overly prominent.
- In iron-deficiency anemia associated with chronic blood loss, erythrocytes are smaller (microcytic) and appear lighter (hypochromic) than normal cells.
- Mild to moderate thrombocytopenia (>30 × 103/µL) does not usually result in spontaneous bleeding, although patients with a pre-existing lesion may bleed in the presence of even mild thrombocytopenia.
- Platelet count may rise in response to significant gastrointestinal bleeding and may fall with multiple blood transfusions.
- Low ferritin level is the most specific test for iron-deficiency anemia. This finding together with a low iron and high TIBC levels are helpful in diagnosing iron-deficiency anemia, a common complication of ongoing or significant UGIB.
- BUN level may be elevated out of proportion to any increase in the creatinine level in patients with UGIB, secondary to breakdown of blood proteins to urea by intestinal bacteria.
- In patients with esophageal varices, acquired coagulopathies are common due to cirrhosis.
Naso-Gastric Lavage
- Nasogastric lavage is only indicated when the diagnosis of UGIB doubtful.[71][72]
- It is rarely used now
- Nasogastric lavage also helps in documenting active or recent UGIB and the need for urgent endoscopy.
- Occasionally used to empty gastric contents in preparation for endoscopy.
Complicatiions
Complications of the procedure include:
- Bleeding from trauma during tube passage in patients with coagulopathy is a possible complication.
- Other rare complications include
- Pharyngeal and esophageal perforation
- Cardiac arrest
- Ethmoid sinus fracture with brain trauma
- Bronchial intubation.
Interpretation
- Evidence of old (brown colored or 'coffee grounds') or fresh blood documents presence of UGIB.
- Evidence of bilious material rules out bleeding distal to the pylorus.
- Any other appearances of GI contents are non-diagnostic.
- There is no evidence that performing a nasogastric lavage to clear clots or otherwise manage bleeding improves clinical outcome.
Contraindications
- Avoid gastric lavage in patients with suspected perforated abdominal viscus.
Upper GI Endoscopy
- Upper GI Endoscopy is considered investigation of choice for diagnosing and assessing the source of UGIB.[73][74][75]
- The American Society of Gastrointestinal Endoscopy guidelines recommend that upper gastrointestinal endoscopy be performed within 24 hours of presentation in all patients with UGIB
Indications
- Active UGIB
- Used for biopsy lesions for tissue diagnosis and to treat currently bleeding lesions.
Complications
Complications include
- Aspiration
- Esophageal perforation
- Cardiopulmonary complications secondary to anesthesia
- Increased bleeding while attempting therapeutic intervention
| If upper GI Endoscopy undiagnostic[33] | |||||||||||||||||||||||||
| Patient’s hemodynamic stability | |||||||||||||||||||||||||
| Stable with low volume bleeding | Unstable with large volume bleeding | ||||||||||||||||||||||||
| Repeat endoscopy | Surgery exploration and partial gastrectomy[76] | ||||||||||||||||||||||||
Other Diagnostic studies
In cases where the source of bleeding is unidentified after upper endoscopy, the utilization of subsequent diagnostic modalities depends upon the hemodynamic stability of the patient. Other diagnostic studies include:[77][78][79]
- CT angiography
- Catheter angiography
- Radionuclide imaging
| CT angiography | Catheter angiography | Radionuclide imaging | |
|---|---|---|---|
| Bleeding at rates
detection |
At least 0.5 mL/min | 0.5 to 1.5 mL/min | 0.1 mL/min |
| Indications |
|
|
|
| Advantages |
|
|
|
| Disadvantages |
|
|
|
Differentiating UGIB
Blood that originates from the oro-pharynx, if swallowed, can present as melena, leading to a false concern of a gastrointestinal source. Examination of nasal area and mouth will help to identify source of bleeding.[80][81][82][83][84][85][86]
| History | Physical Examination | Laboratory Results | |
|---|---|---|---|
| Peptic ulcer disease |
|
|
|
| Mallory-Weiss tear |
|
|
|
| Stress gastritis |
|
|
|
| Dieulafoy lesion |
|
|
|
| Gastro-esophageal
varices |
|
|
|
| Gastric cancer |
|
|
|
| Hemobilia |
|
|
|
| Aortoduodenal
fistula |
|
|
|
Management
The management of GI bleeding includes
- Initial resuscitation and pharmacotherapy
- Risk stratification
- Surgery
- Pre-endoscopy management
- Initial management of antithrombotic agents (anticoagulants and antiplatelet agents)
- Pharmacological therapy
- Role of gastric lavage and prophylactic endotracheal intubation
- Timing of endoscopy
- Endoscopic management
- Endoscopic diagnosis
- Endoscopic therapy
- Pre-endoscopy management
- Management following endoscopy/endoscopic hemostasis
Initial resuscitation
- The initial steps in the management of a patient with UGIB is to assess the severity of bleeding, and then institute fluid and blood resuscitation as needed.[87][88][17]
- Any patient with hemodynamic instability or who is actively bleeding should be admitted to the ICU for monitoring and resuscitation
- The patient’s hemodynamic status is of great importance in determining the degree of severity and triage status.
|
Criteria for Admission of patient |
|---|
|
- The rate of fluid resuscitation is proportional to the severity of bleeding with the goal of restoring and maintaining the patient’s blood pressure.
- Two large caliber (16-gauge or larger) peripheral catheters or a central venous line should be inserted in patients who are hemodynamically unstable. *Supportive care includes administration of supplemental oxygen and monitoring of urine output.
- Patients with severe bleeding will need to be transfused; the indications for transfusion in patients with less severe bleeding should be based on the patient’s age and presence of comorbid conditions.
- The target hematocrit value varies according to age and clinical conditions.
- In the elderly patient, the target hematocrit is 30%.
- In younger, healthy patients, the target hematocrit is 25%.
- In patients with portal hypertension, the target hematocrit should not be above 27% or 28%, so as not to raise portal venous pressure.
- Fresh frozen plasma, platelets, or both should be given to patients with coagulopathy who are actively bleeding and to those who have received more than 10 units of packed erythrocytes
| WORKUP AND INITIAL TREATMENT
Initial Resuscitation | |||
|---|---|---|---|
Basic ABC
| |||
Ensure patent and protected airway
2 large-bore, peripheral intravenous lines
Consider massive transfusion protocol Resuscitate to a target hemoglobin of 7 mg/dL. Consider Sengstaken-Blakemore tube for control of immediately life-threatening upper GI bleeding |
- The National Institute for Health and Care Excellence (NICE) guidline on blood product management in upper GI bleeding:
- Platelets should only be given if the patient is actively bleeding or hemodynamically unstable and has a platelet count of <50×109/L.
- Fresh frozen plasma should be given if the fibrinogen level is <1 g/L or the prothrombin time (PT) or activated partial thromboplastin time is >1.5 times normal.
- Prothrombin complex should be provided to those on warfarin and actively bleeding.
- Recombinant factor VIIa should only be used when all of the above measures have failed.
Endoscopic intervention
- In UGIB, diagnostic and therapeutic endoscopy may be performed simultaneously.
- Therapeutic upper gastrointestinal endoscopy should be performed in all patients with suspected UGIB to evaluate and possibly treat the source of bleeding.
- The urgency of endoscopy depends on the anticipated source of bleeding, rapidity of blood loss, and hemodynamic stability of the patient.
- Endoscopic intervention should be undertaken within 24 hours, as early intervention is associated with reduced transfusion needs and a decreased length of stay in high-risk patients with nonvariceal bleeding.
Endoscopic procedures
- The most common procedures used to manage bleeding caused by peptic ulcer disease are injection, coagulation (thermal, electric, and argon plasma), and hemostatic clips.
- The most common procedures used to manage esophageal varices are sclerotherapy and variceal band ligation
- There is evidence supporting the use of two different endoscopic procedures, rather than a single procedure to better control bleeding and decrease the incidence of rebleeding
- Other successful methods for controlling bleeding are available when endoscopy fails:
- Balloon tamponade and TIPS are temporizing measures for patients with actively bleeding esophageal varices who cannot be managed endoscopically.
- Emergency surgery for bleeding peptic ulcers that cannot be managed endoscopically involves oversewing of the ulcer to ligate the bleeding artery plus truncal vagotomy to decrease acid secretion and pyloroplasty to improve gastric drainage.
Endoscopic band ligation (EBL)
- EBL involves the placement of elastic circular ring ligatures around the varices to cause strangulation, while the patient is under sedation and analgesia. *Bands are typically delivered at the gastroesophageal junction first, then proximally; six to ten bands may be delivered with a single intubation.
- The primary drawback of EBL is that during active bleeding, operator visibility is limited by the device holding the bands prior to their delivery. *Endotracheal intubation is prudent in patients with active bleeding to reduce the risk of aspiration pneumonia.
- Systemic antibiotics should be considered in patients with ascites to reduce the risk of bacterial infection
- Follow-up endoscopies are recommended at various intervals depending on the size/appearance of varices and severity of liver disease.
- Typically, visits every 2 to 4 weeks until obliteration. An interval of 1 to 3 months is recommended for initial surveillance of recurrence of varices, then every 6 to 12 months
- Endoscopic therapy can halt bleeding in 80% to 90% of patients
- EBL is equivalent to EIS in establishing initial control of bleeding, but EBL is challenging in the actively bleeding patient
- EBL is widely favored over EIS for primary prevention due to similar or superior efficacy with fewer complications
Endoscopic injection sclerotherapy (EIS)
- Comprises endoscopic delivery of a sclerosant, such as ethanol, morrhuate sodium, polidocanol, or sodium tetradecyl sulfate, while patient is under sedation and analgesia.
- Injections may be intravariceal or be delivered into the esophageal wall near the varices.
- Bucrylate is an adhesive that has been used successfully.
- Typical injection volume is 1 to 2 mL per injection, for a total volume of 10 to 15 mL. Interval between injections varies according to patient tolerance and response, and complications
- After an initial injection to control bleeding, there is usually a follow-up injection 2 to 3 days later, followed by weekly or biweekly procedures until complete obliteration of the varices is achieved, which usually takes five or six sessions
| Acute GI bleeding | |||||||||||||||||||||||||||||||||||||||||||||||
| Initial evaluation and resuscitation | |||||||||||||||||||||||||||||||||||||||||||||||
| Uppe GI endoscopy | |||||||||||||||||||||||||||||||||||||||||||||||
| Source found | Undiagnostic | ||||||||||||||||||||||||||||||||||||||||||||||
| Specific Treatment | |||||||||||||||||||||||||||||||||||||||||||||||
| Unstable | Stable | ||||||||||||||||||||||||||||||||||||||||||||||
| Urgent CT | Repeat Endoscopy/Angiograpghy | ||||||||||||||||||||||||||||||||||||||||||||||
| No source identified | |||||||||||||||||||||||||||||||||||||||||||||||
| Angioembolization | Endoscopic intervention | TIPS | Surgery | ||||||||||||||||||||||||||||||||||||||||||||
| Surgery (Laprotomy) | |||||||||||||||||||||||||||||||||||||||||||||||
| Sclerotherapy | Banding | Injection | Thermocoagulation | Clips | |||||||||||||||||||||||||||||||||||||||||||
Pharmacotherapy
| Correlation between physical signs and
the severity of upper gastrointestinal bleeding | |||
|---|---|---|---|
| Physical signs | Bleeding severity | ||
| Mild | Moderate | Severe | |
| Blood loss | <1L | 1-2L | >2L |
| Systolic blood pressure | <120 | 100-119 | <99 |
| Orthostasis | - | - | + |
| Tachycardia | <100 | 101-120 | >140 |
| Skin | Warm, well perfused | Diaphoretic | Cool–cold, clammy |
| Urine output(ml/hour) | >25 | 10-25 | Negligible |
| Respiratory rate | 14-20 | 20-30 | >35 |
| Sensorium | Alert | Anxious | Confused/Drowsy |
Physical examination
Common physical exam findings include:
Vitals
- Hypotension
- Tachycardia
- Thready pulse
- Hypoxia
Abdomen
- +Bowel sounds
- Abdominal tenderness
- Hepatomegaly
- Splenomegaly
- Caput medusa
- Spider angiomata
Skin
- Palmar erythema
- Cold clammy extremities
Neurological examination
- Altered sensations
- Poor mentation
- Drowsiness
Rectal examination
- Occult blood
- Gross blood
- Bright red blood per rectum
- Melena
- Burgundy stools
- Blood coating stools versus within stools
- Bloody diarrhea
Surgery
| Surgical options for upper GI bleeding | |
|---|---|
| Disease Process | Surgical Options |
| Peptic ulcer disease | Oversew |
| 3-point ligation of gastroduodenal artery | |
| Vagotomy and pyloroplasty | |
| Vagotomy and antrectomy | |
| Highly selective vagotomy | |
| Mallory-Weiss tear | Oversew |
| Dieulafoy lesion | Oversew |
| Wedge resection | |
| Varices | Portacaval shunt |
| Mesocaval shunt | |
| Distal splenorenal shunt | |
| Gastric cancer | Distal gastrectomy |
| Total gastrectomy | |
| D2 lymphadenectomy | |
| Hemobilia | Selective ligation |
| Resection of aneurysm | |
| Nonselective ligation | |
| Liver resection | |
| Aortoduodenal fistula | Angiography and stent (if hemodynamically stable) |
| Open repair | |
| Extra-anatomic bypass | |
TIPS
TIPS is a complex nonsurgical shunt which involves insertion of an expandable metal stent that bridges the hepatic vein and an intrahepatic branch of the portal vein. TIPS can halt bleeding in almost all patients, including those with bleeding refractory to other therapies.
Indications
- For treatment of bleeding varices that are refractory to banding or sclerosant injection.
- For treatment of refractory variceal bleeding as a bridge to liver transplantation.
Procedure
- TIPS involves the percutaneous puncture of the right internal jugular vein and insertion of a vascular sheath into the inferior vena cava and the hepatic vein.
- A needle is inserted through the sheath, into the liver parenchyma, and then into the portal vein.
- Aspiration of blood and injection of contrast media ensure accurate placement.
- An angioplasty balloon catheter is used to dilate the tract between the hepatic and portal veins, and a stent is then placed across the tract.
- Portal venography is used to confirm the placement
- Patients should be monitored closely for bleeding for 12 to 24 hours
Complications
- Hepatic encephalopathy
- Hemolytic anemia
- Intra-abdominal bleeding during stent placement
Balloon tamponade
Balloon tamponade is only used as a temporary measure in patients who fail to respond to pharmacologic and endoscopic intervention. Balloon tamponade stabilizes patients until more definitive treatment can be instituted (TIPS or liver transplantation).
Procedure
- Balloon tamponade involves the passage of a specialized nasogastric tube, fitted with an inflatable balloon.
- When the balloon is inflated, direct pressure staunches bleeding by compressing the varices.
- Controls active bleeding in 80% to 90% of patients although rebleeding after balloon deflation is common.
Indications
- For bleeding varices that are refractory to banding or sclerosant injection.
Complications
- Rebleeding upon balloon deflation
- Esophageal rupture
Emergency laparotomy
Emergency laparotomy is performed as a last resort for complications such as bleeding and perforation. Emergency laparotomy involving open exploration of the abdomen, oversewing of the ulcer (to ligate the bleeding artery), plus truncal vagotomy (to decrease acid secretion) and pyloroplasty (for improved gastric drainage).
Indications
- Treatment of bleeding ulcer that cannot be managed with endoscopy
- Treatment of patients who cannot tolerate endoscopy
Complications
- Risks of major surgery and general anesthesia
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
 |
|
 |
 |

|
 |
 |

|
The following is a list of diseases that present with acute onset severe lower abdominal pain:
| Disease | Findings |
|---|---|
| Ectopic pregnancy | History of missed menses, positive pregnancy test, ultrasound reveals an empty uterus and may show a mass in the fallopian tubes.[89] |
| Appendicitis | Pain localized to the right iliac fossa, vomiting, abdominal ultrasound sensitivity for diagnosis of acute appendicitis is 75% to 90%.[90] |
| Rupturedovarian cyst | Usually spontaneous, can follow history of trauma, mild chronic lower abdominal discomfort may suddenly intensify, ultrasound is diagnostic.[91] |
| Ovarian cyst torsion | Presents with acute severe unilateral lower quadrant abdominal pain, nausea and vomiting, tender adnexal mass palpated in 90%, ultrasound is diagnostic.[92] |
| Hemorrhagic ovarian cyst | Presents with localized abdominal pain, nausea and vomiting. Hypovolemic shock may be present, abdominal tenderness and guarding are physical exam findings, ultrasound is diagnostic.[92] |
| Endometriosis | Presents with cyclic pain that is exacerbated by onset of menses, dyspareunia. laparoscopic exploration is diagnostic.[92] |
| Acute cystitis | Presents with features of increased urinary frequency, urgency, dysuria, and suprapubic pain.[93][94] |
Diagnosis
- The presence of renal tubular acidosis (RTA) should be considered in any patient with an otherwise unexplained normal anion gap (hyperchloremic) metabolic acidosis.
- The first step in the diagnosis of a patient with a reduced serum bicarbonate and elevated chloride concentration is to confirm that metabolic acidosis is present by measuring the blood pH.
- The next steps in the diagnosis of possible RTA in patients who have a normal anion gap metabolic acidosis are measurement of the urine pH and estimation of urinary ammonium excretion.
Urine PH
- Patients with normal renal function and normal renal acidification mechanisms who develop metabolic acidosis usually have a urine pH of 5.3 or less.
- In most cases of distal RTA, the urine pH is persistently 5.5 or higher, reflecting the primary defect in distal acidification, and a urine pH below 5.5 generally excludes distal (but not proximal) RTA.
- However, the urine pH can be reduced below 5.5 in occasional patients (2 of 17 in one study) with distal RTA.
- In contrast to the persistently elevated urine pH in distal RTA, the urine pH is variable in proximal RTA, a disorder characterized by diminished proximal bicarbonate reabsorption.
- The urine pH will be inappropriately elevated if patients with proximal RTA are treated with alkali, increasing the serum bicarbonate concentration enough to produce a filtered bicarbonate load that exceeds the reduced proximal reabsorptive capacity; this most commonly occurs when alkali is given for the diagnosis or treatment of this disorder.
- In patients presenting with a normal anion gap metabolic acidosis, two scenarios can produce a misleading elevation in the urine pH that incorrectly suggests the presence of RTA:
- Urinary tract infections with urea-splitting organisms may increase the urine pH because urea is converted to ammonia and bicarbonate.
- Thus, assessment of the urine pH should include a urinalysis and, if indicated, a urine culture.
- Severe volume depletion (which indirectly and reversibly limits hydrogen ion secretion by reducing distal sodium delivery) can impair urine acidification.
- Thus, reliable interpretation of an inappropriately high urine pH requires that the urine sodium concentration be greater than 25 meq/L.
- Urinary tract infections with urea-splitting organisms may increase the urine pH because urea is converted to ammonia and bicarbonate.
Urine ammonium excretion
- Urine ammonium excretion is reduced in distal RTA Thus, either direct measurement or indirect estimation of the urine ammonium concentration can be helpful in establishing the correct diagnosis.
- Urinary NH4 excretion cannot be directly measured in most clinical laboratories. However, an indirect estimate can be obtained by measurement of the urine anion gap and/or the urine osmolal gap.
- Estimation of NH4 excretion is not useful in patients with proximal RTA.
References
- ↑ 1.0 1.1 Drini M (2017). "Peptic ulcer disease and non-steroidal anti-inflammatory drugs". Aust Prescr. 40 (3): 91–93. doi:10.18773/austprescr.2017.037. PMC 5478398. PMID 28798512.
- ↑ Pilotto A, Franceschi M, Leandro G, Paris F, Niro V, Longo MG, D'Ambrosio LP, Andriulli A, Di Mario F (2003). "The risk of upper gastrointestinal bleeding in elderly users of aspirin and other non-steroidal anti-inflammatory drugs: the role of gastroprotective drugs". Aging Clin Exp Res. 15 (6): 494–9. PMID 14959953.
- ↑ Hreinsson JP, Kalaitzakis E, Gudmundsson S, Björnsson ES (2013). "Upper gastrointestinal bleeding: incidence, etiology and outcomes in a population-based setting". Scand. J. Gastroenterol. 48 (4): 439–47. doi:10.3109/00365521.2012.763174. PMC 3613943. PMID 23356751.
- ↑ Kaviani MJ, Pirastehfar M, Azari A, Saberifiroozi M (2010). "Etiology and outcome of patients with upper gastrointestinal bleeding: a study from South of Iran". Saudi J Gastroenterol. 16 (4): 253–9. doi:10.4103/1319-3767.70608. PMC 2995092. PMID 20871188.
- ↑ Davidson AT (1985). "Upper gastrointestinal bleeding: causes and treatment". J Natl Med Assoc. 77 (11): 944–5. PMC 2571206. PMID 4078920.
- ↑ 6.0 6.1 6.2 van Leerdam ME (2008). "Epidemiology of acute upper gastrointestinal bleeding". Best Pract Res Clin Gastroenterol. 22 (2): 209–24. doi:10.1016/j.bpg.2007.10.011. PMID 18346679.
- ↑ Morales Uribe CH, Sierra Sierra S, Hernández Hernández AM, Arango Durango AF, López GA (2011). "Upper gastrointestinal bleeding: risk factors for mortality in two urban centres in Latin America". Rev Esp Enferm Dig. 103 (1): 20–4. PMID 21341933.
- ↑ Rodríguez-Hernández H, Rodríguez-Morán M, González JL, Jáquez-Quintana JO, Rodríguez-Acosta ED, Sosa-Tinoco E, Guerrero-Romero F (2009). "[Risk factors associated with upper gastrointestinal bleeding and with mortality]". Rev Med Inst Mex Seguro Soc (in Spanish; Castilian). 47 (2): 179–84. PMID 19744387.
- ↑ Corzo Maldonado MA, Guzmán Rojas P, Bravo Paredes EA, Gallegos López RC, Huerta Mercado-Tenorio J, Surco Ochoa Y, Prochazka Zárate R, Piscoya Rivera A, Pinto Valdivia J, De los Ríos Senmache R (2013). "[Risk factors associated to mortality by upper GI bleeding in patients from a public hospital. A case control study]". Rev Gastroenterol Peru (in Spanish; Castilian). 33 (3): 223–9. PMID 24108375.
- ↑ Soldatov IB, Tokman AS, Esipovich I (1967). "[On the forms of dissemination of advanced experience of otorhinolaryngologists in dispensary work]". Zdravookhr Ross Fed (in Russian). 11 (4): 19–21. PMID 5192276. Vancouver style error: initials (help)
- ↑ Hudzik B, Wilczek K, Gasior M (2016). "Heyde syndrome: gastrointestinal bleeding and aortic stenosis". CMAJ. 188 (2): 135–8. doi:10.1503/cmaj.150194. PMC 4732965. PMID 26124230.
- ↑ Kim BS, Li BT, Engel A, Samra JS, Clarke S, Norton ID, Li AE (2014). "Diagnosis of gastrointestinal bleeding: A practical guide for clinicians". World J Gastrointest Pathophysiol. 5 (4): 467–78. doi:10.4291/wjgp.v5.i4.467. PMC 4231512. PMID 25400991.
- ↑ 13.0 13.1 13.2 Bull-Henry K, Al-Kawas FH (2013). "Evaluation of occult gastrointestinal bleeding". Am Fam Physician. 87 (6): 430–6. PMID 23547576.
- ↑ Jafar W, Jafar A, Sharma A (2016). "Upper gastrointestinal haemorrhage: an update". Frontline Gastroenterol. 7 (1): 32–40. doi:10.1136/flgastro-2014-100492. PMC 5369541. PMID 28839832. Vancouver style error: initials (help)
- ↑ Palmer K (2007). "Acute upper gastrointestinal haemorrhage". Br. Med. Bull. 83: 307–24. doi:10.1093/bmb/ldm023. PMID 17942452.
- ↑ Lau JY, Chung S (2000). "Management of upper gastrointestinal haemorrhage". J. Gastroenterol. Hepatol. 15 Suppl: G8–12. PMID 11100986.
- ↑ 17.0 17.1 Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, Rotondano G, Hucl T, Dinis-Ribeiro M, Marmo R, Racz I, Arezzo A, Hoffmann RT, Lesur G, de Franchis R, Aabakken L, Veitch A, Radaelli F, Salgueiro P, Cardoso R, Maia L, Zullo A, Cipolletta L, Hassan C (2015). "Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) Guideline". Endoscopy. 47 (10): a1–46. doi:10.1055/s-0034-1393172. PMID 26417980.
- ↑ Brooks J, Warburton R, Beales IL (2013). "Prevention of upper gastrointestinal haemorrhage: current controversies and clinical guidance". Ther Adv Chronic Dis. 4 (5): 206–22. doi:10.1177/2040622313492188. PMC 3752180. PMID 23997925.
- ↑ Yasuda H, Matsuo Y, Sato Y, Ozawa S, Ishigooka S, Yamashita M, Yamamoto H, Itoh F (2015). "Treatment and prevention of gastrointestinal bleeding in patients receiving antiplatelet therapy". World J Crit Care Med. 4 (1): 40–6. doi:10.5492/wjccm.v4.i1.40. PMC 4326762. PMID 25685721.
- ↑ Biecker E, Heller J, Schmitz V, Lammert F, Sauerbruch T (2008). "Diagnosis and management of upper gastrointestinal bleeding". Dtsch Arztebl Int. 105 (5): 85–94. doi:10.3238/arztebl.2008.0085. PMC 2701242. PMID 19633792.
- ↑ Chan FK (2012). "Anti-platelet therapy and managing ulcer risk". J. Gastroenterol. Hepatol. 27 (2): 195–9. doi:10.1111/j.1440-1746.2011.07029.x. PMID 22142030.
- ↑ Garcia-Tsao, Guadalupe; Sanyal, Arun J.; Grace, Norman D.; Carey, William D. (2007). "Prevention and Management of Gastroesophageal Varices and Variceal Hemorrhage in Cirrhosis". The American Journal of Gastroenterology. 102 (9): 2086–2102. doi:10.1111/j.1572-0241.2007.01481.x. ISSN 0002-9270.
- ↑ Roberts SE, Button LA, Williams JG (2012). "Prognosis following upper gastrointestinal bleeding". PLoS ONE. 7 (12): e49507. doi:10.1371/journal.pone.0049507. PMC 3520969. PMID 23251344.
- ↑ Katschinski B, Logan R, Davies J, Faulkner G, Pearson J, Langman M (1994). "Prognostic factors in upper gastrointestinal bleeding". Dig. Dis. Sci. 39 (4): 706–12. PMID 7908623.
- ↑ Kurien M, Lobo AJ (2015). "Acute upper gastrointestinal bleeding". Clin Med (Lond). 15 (5): 481–5. doi:10.7861/clinmedicine.15-5-481. PMID 26430191.
- ↑ Feinman M, Haut ER (2014). "Upper gastrointestinal bleeding". Surg. Clin. North Am. 94 (1): 43–53. doi:10.1016/j.suc.2013.10.004. PMID 24267496.
- ↑ Ebrahimi Bakhtavar H, Morteza Bagi HR, Rahmani F, Shahsavari Nia K, Ettehadi A (2017). "Clinical Scoring Systems in Predicting the Outcome of Acute Upper Gastrointestinal Bleeding; a Narrative Review". Emerg (Tehran). 5 (1): e36. PMC 5325906. PMID 28286843.
- ↑ Blatchford O, Murray WR, Blatchford M (2000). "A risk score to predict need for treatment for upper-gastrointestinal haemorrhage". Lancet. 356 (9238): 1318–21. doi:10.1016/S0140-6736(00)02816-6. PMID 11073021.
- ↑ Stanley AJ (2012). "Update on risk scoring systems for patients with upper gastrointestinal haemorrhage". World J. Gastroenterol. 18 (22): 2739–44. doi:10.3748/wjg.v18.i22.2739. PMC 3374976. PMID 22719181.
- ↑ Monteiro S, Gonçalves TC, Magalhães J, Cotter J (2016). "Upper gastrointestinal bleeding risk scores: Who, when and why?". World J Gastrointest Pathophysiol. 7 (1): 86–96. doi:10.4291/wjgp.v7.i1.86. PMC 4753192.
- ↑ Atkinson RJ, Hurlstone DP (2008). "Usefulness of prognostic indices in upper gastrointestinal bleeding". Best Pract Res Clin Gastroenterol. 22 (2): 233–42. doi:10.1016/j.bpg.2007.11.004. PMID 18346681.
- ↑ Sonnenberg A (2012). "Complications following gastrointestinal bleeding and their impact on outcome and death". Eur J Gastroenterol Hepatol. 24 (4): 388–92. doi:10.1097/MEG.0b013e328350589e. PMID 22233622.
- ↑ 33.0 33.1 "Non-variceal upper gastrointestinal haemorrhage: guidelines". Gut. 51 Suppl 4: iv1–6. 2002. PMC 1867732. PMID 12208839.
- ↑ 34.0 34.1 Raju GS, Gerson L, Das A, Lewis B (2007). "American Gastroenterological Association (AGA) Institute medical position statement on obscure gastrointestinal bleeding". Gastroenterology. 133 (5): 1694–6. doi:10.1053/j.gastro.2007.06.008. PMID 17983811.
- ↑ Rockey DC (1999). "Occult gastrointestinal bleeding". N. Engl. J. Med. 341 (1): 38–46. doi:10.1056/NEJM199907013410107. PMID 10387941.
- ↑ El-Tawil AM (2012). "Trends on gastrointestinal bleeding and mortality: where are we standing?". World J. Gastroenterol. 18 (11): 1154–8. doi:10.3748/wjg.v18.i11.1154. PMC 3309903. PMID 22468077.
- ↑ Feldman SE (1970). "Blood supply to stomach". Calif Med. 112 (4): 55. PMC 1501289. PMID 18730308.
- ↑ Granger DN, Holm L, Kvietys P (2015). "The Gastrointestinal Circulation: Physiology and Pathophysiology". Compr Physiol. 5 (3): 1541–83. doi:10.1002/cphy.c150007. PMID 26140727.
- ↑ Geboes K, Geboes KP, Maleux G (2001). "Vascular anatomy of the gastrointestinal tract". Best Pract Res Clin Gastroenterol. 15 (1): 1–14. doi:10.1053/bega.2000.0152. PMID 11355897.
- ↑ Varga F, Csáky TZ (1976). "Changes in the blood supply of the gastrointestinal tract in rats with age". Pflugers Arch. 364 (2): 129–33. PMID 986621.
- ↑ Matuchansky C, Bernier JJ (1973). "[Prostaglandins and the digestive tract]". Biol Gastroenterol (Paris) (in French). 6 (3): 251–68. PMID 4599528.
- ↑ Radbil' OS (1974). "[Prostaglandins and the digestive system organs]". Ter. Arkh. (in Russian). 46 (4): 6–14. PMID 4372738.
- ↑ Robert A (1980). "Prostaglandins and digestive diseases". Adv Prostaglandin Thromboxane Res. 8: 1533–41. PMID 6990725.
- ↑ Hills BA, Butler BD, Lichtenberger LM (1983). "Gastric mucosal barrier: hydrophobic lining to the lumen of the stomach". Am. J. Physiol. 244 (5): G561–8. PMID 6846549.
- ↑ Clamp JR, Ene D (1989). "The gastric mucosal barrier". Methods Find Exp Clin Pharmacol. 11 Suppl 1: 19–25. PMID 2657286.
- ↑ Werther JL (2000). "The gastric mucosal barrier". Mt. Sinai J. Med. 67 (1): 41–53. PMID 10677782.
- ↑ Forssell H (1988). "Gastric mucosal defence mechanisms: a brief review". Scand. J. Gastroenterol. Suppl. 155: 23–8. PMID 3072665.
- ↑ Boonpongmanee S, Fleischer DE, Pezzullo JC, Collier K, Mayoral W, Al-Kawas F, Chutkan R, Lewis JH, Tio TL, Benjamin SB (2004). "The frequency of peptic ulcer as a cause of upper-GI bleeding is exaggerated". Gastrointest. Endosc. 59 (7): 788–94. PMID 15173790.
- ↑ Gartner AH (1976). "Aspirin-induced gastritis and gastrointestinal bleeding". J Am Dent Assoc. 93 (1): 111–7. PMID 6499.
- ↑ Iwamoto J, Saito Y, Honda A, Matsuzaki Y (2013). "Clinical features of gastroduodenal injury associated with long-term low-dose aspirin therapy". World J. Gastroenterol. 19 (11): 1673–82. doi:10.3748/wjg.v19.i11.1673. PMC 3607744. PMID 23555156.
- ↑ Hawkey CJ (1996). "Non-steroidal anti-inflammatory drug gastropathy: causes and treatment". Scand. J. Gastroenterol. Suppl. 220: 124–7. PMID 8898449.
- ↑ Quan S, Yang H, Tanyingoh D, Villeneuve PJ, Stieb DM, Johnson M, Hilsden R, Madsen K, van Zanten SV, Novak K, Lang E, Ghosh S, Kaplan GG (2015). "Upper gastrointestinal bleeding due to peptic ulcer disease is not associated with air pollution: a case-crossover study". BMC Gastroenterol. 15: 131. doi:10.1186/s12876-015-0363-6. PMC 4604641. PMID 26467538.
- ↑ Quan, C (2002). "Management of peptic ulcer disease not related to Helicobacter pylori or NSAIDs". The American Journal of Gastroenterology. 97 (12): 2950–2961. doi:10.1016/S0002-9270(02)05485-0. ISSN 0002-9270.
- ↑ Malfertheiner, Peter; Chan, Francis KL; McColl, Kenneth EL (2009). "Peptic ulcer disease". The Lancet. 374 (9699): 1449–1461. doi:10.1016/S0140-6736(09)60938-7. ISSN 0140-6736.
- ↑ Quan S, Frolkis A, Milne K, Molodecky N, Yang H, Dixon E, Ball CG, Myers RP, Ghosh S, Hilsden R, van Zanten SV, Kaplan GG (2014). "Upper-gastrointestinal bleeding secondary to peptic ulcer disease: incidence and outcomes". World J. Gastroenterol. 20 (46): 17568–77. doi:10.3748/wjg.v20.i46.17568. PMC 4265619. PMID 25516672.
- ↑ Xi B, Jia JJ, Lin BY, Geng L, Zheng SS (2016). "Peptic ulcers accompanied with gastrointestinal bleeding, pylorus obstruction and cholangitis secondary to choledochoduodenal fistula: A case report". Oncol Lett. 11 (1): 481–483. doi:10.3892/ol.2015.3908. PMC 4727103. PMID 26870237.
- ↑ Stern AI, Korman MG, Hunt PS, Hansky J, Hillman HS, Schmidt GT (1979). "The Mallory-Weiss lesion as a cause of upper gastrointestinal bleeding". Aust N Z J Surg. 49 (1): 13–8. PMID 313784.
- ↑ Katz PO, Salas L (1993). "Less frequent causes of upper gastrointestinal bleeding". Gastroenterol. Clin. North Am. 22 (4): 875–89. PMID 8307643.
- ↑ Sabljak P, Velicković D, Stojakov D, Bjelović M, Ebrahimi K, Spica B, Sljukić V, Pesko P (2007). "[Less frequent causes of upper gastrointestinal bleeding]". Acta Chir Iugosl. 54 (1): 119–23. PMID 17633871.
- ↑ Depolo A, Dobrila-Dintinjana R, Uravi M, Grbas H, Rubini M (2001). "[Upper gastrointestinal bleeding - Review of our ten years results]". Zentralbl Chir (in German). 126 (10): 772–6. doi:10.1055/s-2001-18265. PMID 11727185.
- ↑ 61.0 61.1 Renoult E, Biava MF, Aimone-Gastin I, Aouragh F, Hestin D, Kures L, Kessler M (1992). "Evolution and significance of Toxoplasma gondii antibody titers in kidney transplant recipients". Transplant. Proc. 24 (6): 2754–5. PMID 1465928.
- ↑ Rosołowski M, Kierzkiewicz M (2013). "Etiology, diagnosis and treatment of infectious esophagitis". Prz Gastroenterol. 8 (6): 333–7. doi:10.5114/pg.2013.39914. PMC 4027832. PMID 24868280.
- ↑ Pandit S, Boktor M, Alexander JS, Becker F, Morris J (2017). "Gastroesophageal reflux disease: A clinical overview for primary care physicians". Pathophysiology. doi:10.1016/j.pathophys.2017.09.001. PMID 28943113.
- ↑ Rajendra S, Sharma P (2017). "Barrett Esophagus and Intramucosal Esophageal Adenocarcinoma". Hematol. Oncol. Clin. North Am. 31 (3): 409–426. doi:10.1016/j.hoc.2017.01.003. PMID 28501084.
- ↑ 65.0 65.1 Garg H, Gupta S, Anand AC, Broor SL (2015). "Portal hypertensive gastropathy and gastric antral vascular ectasia". Indian J Gastroenterol. 34 (5): 351–8. doi:10.1007/s12664-015-0605-0. PMID 26564121.
- ↑ Gordon FH, Watkinson A, Hodgson H (2001). "Vascular malformations of the gastrointestinal tract". Best Pract Res Clin Gastroenterol. 15 (1): 41–58. doi:10.1053/bega.2000.0155. PMID 11355900.
- ↑ Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, Sinclair P (2010). "International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding". Ann. Intern. Med. 152 (2): 101–13. doi:10.7326/0003-4819-152-2-201001190-00009. PMID 20083829.
- ↑ Hussain H, Lapin S, Cappell MS (2000). "Clinical scoring systems for determining the prognosis of gastrointestinal bleeding". Gastroenterol. Clin. North Am. 29 (2): 445–64. PMID 10836189.
- ↑ Stanley AJ, Ashley D, Dalton HR, Mowat C, Gaya DR, Thompson E, Warshow U, Groome M, Cahill A, Benson G, Blatchford O, Murray W (2009). "Outpatient management of patients with low-risk upper-gastrointestinal haemorrhage: multicentre validation and prospective evaluation". Lancet. 373 (9657): 42–7. doi:10.1016/S0140-6736(08)61769-9. PMID 19091393.
- ↑ Rockall TA, Logan RF, Devlin HB, Northfield TC (1996). "Selection of patients for early discharge or outpatient care after acute upper gastrointestinal haemorrhage. National Audit of Acute Upper Gastrointestinal Haemorrhage". Lancet. 347 (9009): 1138–40. PMID 8609747.
- ↑ Pallin DJ, Saltzman JR (2011). "Is nasogastric tube lavage in patients with acute upper GI bleeding indicated or antiquated?". Gastrointest. Endosc. 74 (5): 981–4. doi:10.1016/j.gie.2011.07.007. PMID 22032314.
- ↑ Marshall JB (1982). "Management of acute upper gastrointestinal bleeding". Postgrad Med. 71 (5): 149–54, 157–8. PMID 6978482.
- ↑ Cappell MS, Friedel D (2002). "The role of esophagogastroduodenoscopy in the diagnosis and management of upper gastrointestinal disorders". Med. Clin. North Am. 86 (6): 1165–216. PMID 12510452.
- ↑ Jaskolka JD, Binkhamis S, Prabhudesai V, Chawla TP (2013). "Acute gastrointestinal hemorrhage: radiologic diagnosis and management". Can Assoc Radiol J. 64 (2): 90–100. doi:10.1016/j.carj.2012.08.001. PMID 23245297.
- ↑ Jensen DM, Kovacs TO, Jutabha R, Machicado GA, Gralnek IM, Savides TJ, Smith J, Jensen ME, Alofaituli G, Gornbein J (2002). "Randomized trial of medical or endoscopic therapy to prevent recurrent ulcer hemorrhage in patients with adherent clots". Gastroenterology. 123 (2): 407–13. PMID 12145792.
- ↑ Zmora O, Dinnewitzer AJ, Pikarsky AJ, Efron JE, Weiss EG, Nogueras JJ, Wexner SD (2002). "Intraoperative endoscopy in laparoscopic colectomy". Surg Endosc. 16 (5): 808–11. doi:10.1007/s00464-001-8226-3. PMID 11997827.
- ↑ Steer ML, Silen W (1983). "Diagnostic procedures in gastrointestinal hemorrhage". N. Engl. J. Med. 309 (11): 646–50. doi:10.1056/NEJM198309153091106. PMID 6604219.
- ↑ Browder W, Cerise EJ, Litwin MS (1986). "Impact of emergency angiography in massive lower gastrointestinal bleeding". Ann. Surg. 204 (5): 530–6. PMC 1251335. PMID 3094466.
- ↑ Hunter JM, Pezim ME (1990). "Limited value of technetium 99m-labeled red cell scintigraphy in localization of lower gastrointestinal bleeding". Am. J. Surg. 159 (5): 504–6. PMID 2334015.
- ↑ Graham DY (2016). "Upper Gastrointestinal Bleeding Due to a Peptic Ulcer". N. Engl. J. Med. 375 (12): 1197–8. doi:10.1056/NEJMc1609017#SA2. PMID 27653583.
- ↑ Chen ZJ, Freeman ML (2011). "Management of upper gastrointestinal bleeding emergencies: evidence-based medicine and practical considerations". World J Emerg Med. 2 (1): 5–12. PMC 4129733. PMID 25214975.
- ↑ Kaufman DW, Kelly JP, Wiholm BE, Laszlo A, Sheehan JE, Koff RS, Shapiro S (1999). "The risk of acute major upper gastrointestinal bleeding among users of aspirin and ibuprofen at various levels of alcohol consumption". Am. J. Gastroenterol. 94 (11): 3189–96. doi:10.1111/j.1572-0241.1999.01517.x. PMID 10566713.
- ↑ Lee EW, Laberge JM (2004). "Differential diagnosis of gastrointestinal bleeding". Tech Vasc Interv Radiol. 7 (3): 112–22. PMID 16015555.
- ↑ Lee YT, Walmsley RS, Leong RW, Sung JJ (2003). "Dieulafoy's lesion". Gastrointest. Endosc. 58 (2): 236–43. doi:10.1067/mge.2003.328. PMID 12872092.
- ↑ Ghosh S, Watts D, Kinnear M (2002). "Management of gastrointestinal haemorrhage". Postgrad Med J. 78 (915): 4–14. PMC 1742226. PMID 11796865.
- ↑ Chalasani N, Clark WS, Wilcox CM (1997). "Blood urea nitrogen to creatinine concentration in gastrointestinal bleeding: a reappraisal". Am. J. Gastroenterol. 92 (10): 1796–9. PMID 9382039.
- ↑ Wassef W (2004). "Upper gastrointestinal bleeding". Curr. Opin. Gastroenterol. 20 (6): 538–45. PMID 15703679.
- ↑ Kovacs TO (2008). "Management of upper gastrointestinal bleeding". Curr Gastroenterol Rep. 10 (6): 535–42. PMID 19006607.
- ↑ Morin L, Cargill YM, Glanc P (2016). "Ultrasound Evaluation of First Trimester Complications of Pregnancy". J Obstet Gynaecol Can. 38 (10): 982–988. doi:10.1016/j.jogc.2016.06.001. PMID 27720100.
- ↑ Balthazar EJ, Birnbaum BA, Yee J, Megibow AJ, Roshkow J, Gray C (1994). "Acute appendicitis: CT and US correlation in 100 patients". Radiology. 190 (1): 31–5. doi:10.1148/radiology.190.1.8259423. PMID 8259423.
- ↑ Bottomley C, Bourne T (2009). "Diagnosis and management of ovarian cyst accidents". Best Pract Res Clin Obstet Gynaecol. 23 (5): 711–24. doi:10.1016/j.bpobgyn.2009.02.001. PMID 19299205.
- ↑ 92.0 92.1 92.2 Bhavsar AK, Gelner EJ, Shorma T (2016). "Common Questions About the Evaluation of Acute Pelvic Pain". Am Fam Physician. 93 (1): 41–8. PMID 26760839.
- ↑ {{Cite journal | author = W. E. Stamm | title = Etiology and management of the acute urethral syndrome | journal = Sexually transmitted diseases | volume = 8 | issue = 3 | pages = 235–238 | year = 1981 | month = July-September | pmid = 7292216
- ↑ {{Cite journal | author = W. E. Stamm, K. F. Wagner, R. Amsel, E. R. Alexander, M. Turck, G. W. Counts & K. K. Holmes | title = Causes of the acute urethral syndrome in women | journal = The New England journal of medicine | volume = 303 | issue = 8 | pages = 409–415 | year = 1980 | month = August | doi = 10.1056/NEJM198008213030801 | pmid = 6993946
References