Rizatriptan: Difference between revisions
No edit summary |
No edit summary |
||
| Line 7: | Line 7: | ||
|genericName= | |genericName= | ||
Rizatriptan | |||
|aOrAn= | |aOrAn= | ||
| Line 15: | Line 15: | ||
|drugClass= | |drugClass= | ||
serotonin (5-HT) 1B/1D receptor agonist | |||
|indication= | |indication= | ||
acute treatment of migraine with or without aura | |||
|hasBlackBoxWarning= | |hasBlackBoxWarning= | ||
|adverseReactions= | |adverseReactions= | ||
asthenia/fatigue, somnolence, pain/pressure sensation and dizziness | |||
<!--Black Box Warning--> | <!--Black Box Warning--> | ||
| Line 45: | Line 43: | ||
|fdaLIADAdult= | |fdaLIADAdult= | ||
===== | =====Migraine===== | ||
* Dosing Information | * Dosing Information | ||
:* | :*The recommended starting dose of MAXALT is either 5 mg or 10 mg for the acute treatment of migraines in adults. The 10-mg dose may provide a greater effect than the 5-mg dose, but may have a greater risk of adverse reactions [see Clinical Studies (14.1)]. | ||
:* | *Redosing in Adults | ||
:*Although the effectiveness of a second dose or subsequent doses has not been established in placebo-controlled trials, if the migraine headache returns, a second dose may be administered 2 hours after the first dose. The maximum daily dose should not exceed 30 mg in any 24-hour period. The safety of treating, on average, more than four headaches in a 30-day period has not been established. | |||
<!--Off-Label Use and Dosage (Adult)--> | <!--Off-Label Use and Dosage (Adult)--> | ||
| Line 74: | Line 57: | ||
|offLabelAdultGuideSupport= | |offLabelAdultGuideSupport= | ||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| Line 94: | Line 63: | ||
|offLabelAdultNoGuideSupport= | |offLabelAdultNoGuideSupport= | ||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | ||
| Line 115: | Line 76: | ||
* Dosing Information | * Dosing Information | ||
:* | :* Dosing in pediatric patients is based on the patient's body weight. The recommended dose of MAXALT is 5 mg in patients weighing less than 40 kg (88 lb), and 10 mg in patients weighing 40 kg (88 lb) or more. | ||
:*The efficacy and safety of treatment with more than one dose of MAXALT within 24 hours in pediatric patients 6 to 17 years of age have not been established. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | <!--Off-Label Use and Dosage (Pediatric)--> | ||
| Line 127: | Line 85: | ||
|offLabelPedGuideSupport= | |offLabelPedGuideSupport= | ||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport= | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications= | |||
* | *Ischemic coronary artery disease (angina pectoris, history of myocardial infarction, or documented silent ischemia), or other significant underlying cardiovascular disease [see Warnings and Precautions (5.1)]. | ||
*Coronary artery vasospasm including Prinzmetal's angina [see Warnings and Precautions (5.1)]. | |||
*History of stroke or transient ischemic attack (TIA) [see Warnings and Precautions (5.4)]. | |||
*Peripheral vascular disease (PVD) [see Warnings and Precautions (5.5)]. | |||
*Ischemic bowel disease [see Warnings and Precautions (5.5)]. | |||
*Uncontrolled hypertension [see Warnings and Precautions (5.8)]. | |||
*Recent use (i.e., within 24 hours) of another 5-HT1 agonist, ergotamine-containing medication, or ergot-type medication (such as dihydroergotamine or methysergide) [see Drug Interactions (7.2 and 7.3)]. | |||
*Hemiplegic or basilar migraine [see Indications and Usage (1)]. | |||
*Concurrent administration or recent discontinuation (i.e., within 2 weeks) of a MAO-A inhibitor [see Drug Interactions (7.5) and Clinical Pharmacology (12.3)]. | |||
* | *Hypersensitivity to MAXALT or MAXALT-MLT (angioedema and anaphylaxis seen) [see Adverse Reactions (6.2)]. | ||
<!--Warnings--> | |||
|warnings= | |||
====Precautions==== | |||
*Myocardial Ischemia, Myocardial Infarction, and Prinzmetal's Angina | |||
:*MAXALT should not be given to patients with ischemic or vasospastic coronary artery disease. There have been rare reports of serious cardiac adverse reactions, including acute myocardial infarction, occurring within a few hours following administration of MAXALT. Some of these reactions occurred in patients without known coronary artery disease (CAD). 5-HT1 agonists, including MAXALT may cause coronary artery vasospasm (Prinzmetal's Angina), even in patients without a history of CAD. | |||
:*Triptan-naïve patients who have multiple cardiovascular risk factors (e.g., increased age, diabetes, hypertension, smoking, obesity, strong family history of CAD) should have a cardiovascular evaluation prior to receiving MAXALT. If there is evidence of CAD or coronary artery vasospasm, MAXALT should not be administered [see Contraindications (4)]. For patients who have a negative cardiovascular evaluation, consideration should be given to administration of the first MAXALT dose in a medically-supervised setting and performing an electrocardiogram (ECG) immediately following MAXALT administration. Periodic cardiovascular evaluation should be considered in intermittent long-term users of MAXALT who have cardiovascular risk factors. | |||
*Arrhythmias | |||
:*Life-threatening disturbances of cardiac rhythm, including ventricular tachycardia and ventricular fibrillation leading to death, have been reported within a few hours following the administration of 5-HT1 agonists. Discontinue MAXALT if these disturbances occur. | |||
* | *Chest, Throat, Neck and/or Jaw Pain/Tightness/Pressure | ||
:*As with other 5-HT1 agonists, sensations of tightness, pain, pressure, and heaviness in the precordium, throat, neck and jaw commonly occur after treatment with MAXALT and are usually non-cardiac in origin. However, if a cardiac origin is suspected, patients should be evaluated. Patients shown to have CAD and those with Prinzmetal's variant angina should not receive 5-HT1 agonists. | |||
*Cerebrovascular Events | |||
:*Cerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT1 agonists, and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the 5-HT1 agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine, when they were not. Also, patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, hemorrhage, transient ischemic attack). Discontinue MAXALT if a cerebrovascular event occurs. | |||
:*As with other acute migraine therapies, before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with atypical symptoms, care should be taken to exclude other potentially serious neurological conditions. MAXALT should not be administered to patients with a history of stroke or transient ischemic attack [see Contraindications (4)]. | |||
*Other Vasospasm Reactions | |||
:*5-HT1 agonists, including MAXALT, may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), splenic infarction, and Raynaud's syndrome. In patients who experience symptoms or signs suggestive of non-coronary vasospasm reaction following the use of any 5-HT1 agonist, the suspected vasospasm reaction should be ruled out before receiving additional MAXALT doses. | |||
:*Reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT1 agonists. Since visual disorders may be part of a migraine attack, a causal relationship between these events and the use of 5-HT1 agonists have not been clearly established. | |||
* | *Medication Overuse Headache | ||
:*Overuse of acute migraine drugs (e.g., ergotamine, triptans, opioids, or a combination of drugs for 10 or more days per month) may lead to exacerbation of headache (medication overuse headache). Medication overuse headache may present as migraine-like daily headaches, or as a marked increase in frequency of migraine attacks. Detoxification of patients, including withdrawal of the overused drugs, and treatment of withdrawal symptoms (which often includes a transient worsening of headache) may be necessary. | |||
*Serotonin Syndrome | |||
:*Serotonin syndrome may occur with triptans, including MAXALT particularly during co-administration with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and MAO inhibitors [see Drug Interactions (7.5)]. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms can occur within minutes to hours of receiving a new or a greater dose of a serotonergic medication. MAXALT treatment should be discontinued if serotonin syndrome is suspected [see Drug Interactions (7.4) and Patient Counseling Information (17)]. | |||
* | *Increase in Blood Pressure | ||
:*Significant elevation in blood pressure, including hypertensive crisis with acute impairment of organ systems, has been reported on rare occasions in patients with and without a history of hypertension receiving 5-HT1 agonists, including MAXALT. In healthy young adult male and female patients who received maximal doses of MAXALT (10 mg every 2 hours for 3 doses), slight increases in blood pressure (approximately 2-3 mmHg) were observed. MAXALT is contraindicated in patients with uncontrolled hypertension [see Contraindications (4)]. | |||
<!--Adverse Reactions--> | <!--Adverse Reactions--> | ||
Revision as of 21:11, 23 October 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
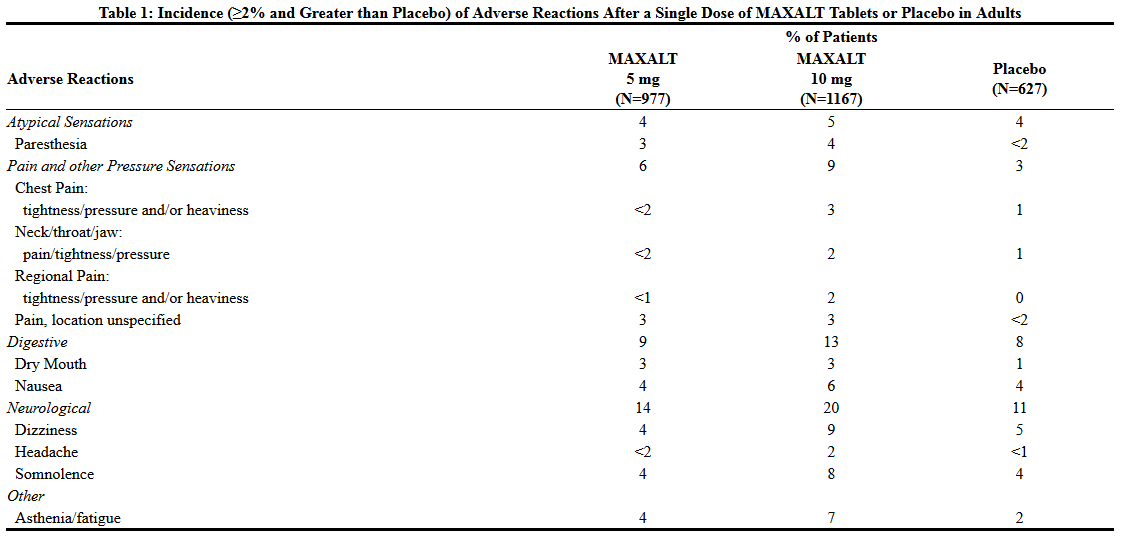
Rizatriptan is a serotonin (5-HT) 1B/1D receptor agonist that is FDA approved for the {{{indicationType}}} of acute treatment of migraine with or without aura. Common adverse reactions include asthenia/fatigue, somnolence, pain/pressure sensation and dizziness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Migraine
- Dosing Information
- The recommended starting dose of MAXALT is either 5 mg or 10 mg for the acute treatment of migraines in adults. The 10-mg dose may provide a greater effect than the 5-mg dose, but may have a greater risk of adverse reactions [see Clinical Studies (14.1)].
- Redosing in Adults
- Although the effectiveness of a second dose or subsequent doses has not been established in placebo-controlled trials, if the migraine headache returns, a second dose may be administered 2 hours after the first dose. The maximum daily dose should not exceed 30 mg in any 24-hour period. The safety of treating, on average, more than four headaches in a 30-day period has not been established.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rizatriptan in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Rizatriptan in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosing in pediatric patients is based on the patient's body weight. The recommended dose of MAXALT is 5 mg in patients weighing less than 40 kg (88 lb), and 10 mg in patients weighing 40 kg (88 lb) or more.
- The efficacy and safety of treatment with more than one dose of MAXALT within 24 hours in pediatric patients 6 to 17 years of age have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rizatriptan in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Rizatriptan in pediatric patients.
Contraindications
- Ischemic coronary artery disease (angina pectoris, history of myocardial infarction, or documented silent ischemia), or other significant underlying cardiovascular disease [see Warnings and Precautions (5.1)].
- Coronary artery vasospasm including Prinzmetal's angina [see Warnings and Precautions (5.1)].
- History of stroke or transient ischemic attack (TIA) [see Warnings and Precautions (5.4)].
- Peripheral vascular disease (PVD) [see Warnings and Precautions (5.5)].
- Ischemic bowel disease [see Warnings and Precautions (5.5)].
- Uncontrolled hypertension [see Warnings and Precautions (5.8)].
- Recent use (i.e., within 24 hours) of another 5-HT1 agonist, ergotamine-containing medication, or ergot-type medication (such as dihydroergotamine or methysergide) [see Drug Interactions (7.2 and 7.3)].
- Hemiplegic or basilar migraine [see Indications and Usage (1)].
- Concurrent administration or recent discontinuation (i.e., within 2 weeks) of a MAO-A inhibitor [see Drug Interactions (7.5) and Clinical Pharmacology (12.3)].
- Hypersensitivity to MAXALT or MAXALT-MLT (angioedema and anaphylaxis seen) [see Adverse Reactions (6.2)].
Warnings
Precautions
- Myocardial Ischemia, Myocardial Infarction, and Prinzmetal's Angina
- MAXALT should not be given to patients with ischemic or vasospastic coronary artery disease. There have been rare reports of serious cardiac adverse reactions, including acute myocardial infarction, occurring within a few hours following administration of MAXALT. Some of these reactions occurred in patients without known coronary artery disease (CAD). 5-HT1 agonists, including MAXALT may cause coronary artery vasospasm (Prinzmetal's Angina), even in patients without a history of CAD.
- Triptan-naïve patients who have multiple cardiovascular risk factors (e.g., increased age, diabetes, hypertension, smoking, obesity, strong family history of CAD) should have a cardiovascular evaluation prior to receiving MAXALT. If there is evidence of CAD or coronary artery vasospasm, MAXALT should not be administered [see Contraindications (4)]. For patients who have a negative cardiovascular evaluation, consideration should be given to administration of the first MAXALT dose in a medically-supervised setting and performing an electrocardiogram (ECG) immediately following MAXALT administration. Periodic cardiovascular evaluation should be considered in intermittent long-term users of MAXALT who have cardiovascular risk factors.
- Arrhythmias
- Life-threatening disturbances of cardiac rhythm, including ventricular tachycardia and ventricular fibrillation leading to death, have been reported within a few hours following the administration of 5-HT1 agonists. Discontinue MAXALT if these disturbances occur.
- Chest, Throat, Neck and/or Jaw Pain/Tightness/Pressure
- As with other 5-HT1 agonists, sensations of tightness, pain, pressure, and heaviness in the precordium, throat, neck and jaw commonly occur after treatment with MAXALT and are usually non-cardiac in origin. However, if a cardiac origin is suspected, patients should be evaluated. Patients shown to have CAD and those with Prinzmetal's variant angina should not receive 5-HT1 agonists.
- Cerebrovascular Events
- Cerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT1 agonists, and some have resulted in fatalities. In a number of cases, it appears possible that the cerebrovascular events were primary, the 5-HT1 agonist having been administered in the incorrect belief that the symptoms experienced were a consequence of migraine, when they were not. Also, patients with migraine may be at increased risk of certain cerebrovascular events (e.g., stroke, hemorrhage, transient ischemic attack). Discontinue MAXALT if a cerebrovascular event occurs.
- As with other acute migraine therapies, before treating headaches in patients not previously diagnosed as migraineurs, and in migraineurs who present with atypical symptoms, care should be taken to exclude other potentially serious neurological conditions. MAXALT should not be administered to patients with a history of stroke or transient ischemic attack [see Contraindications (4)].
- Other Vasospasm Reactions
- 5-HT1 agonists, including MAXALT, may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction (presenting with abdominal pain and bloody diarrhea), splenic infarction, and Raynaud's syndrome. In patients who experience symptoms or signs suggestive of non-coronary vasospasm reaction following the use of any 5-HT1 agonist, the suspected vasospasm reaction should be ruled out before receiving additional MAXALT doses.
- Reports of transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT1 agonists. Since visual disorders may be part of a migraine attack, a causal relationship between these events and the use of 5-HT1 agonists have not been clearly established.
- Medication Overuse Headache
- Overuse of acute migraine drugs (e.g., ergotamine, triptans, opioids, or a combination of drugs for 10 or more days per month) may lead to exacerbation of headache (medication overuse headache). Medication overuse headache may present as migraine-like daily headaches, or as a marked increase in frequency of migraine attacks. Detoxification of patients, including withdrawal of the overused drugs, and treatment of withdrawal symptoms (which often includes a transient worsening of headache) may be necessary.
- Serotonin Syndrome
- Serotonin syndrome may occur with triptans, including MAXALT particularly during co-administration with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and MAO inhibitors [see Drug Interactions (7.5)]. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, coma), autonomic instability (e.g., tachycardia, labile blood pressure, hyperthermia), neuromuscular aberrations (e.g., hyperreflexia, incoordination) and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). The onset of symptoms can occur within minutes to hours of receiving a new or a greater dose of a serotonergic medication. MAXALT treatment should be discontinued if serotonin syndrome is suspected [see Drug Interactions (7.4) and Patient Counseling Information (17)].
- Increase in Blood Pressure
- Significant elevation in blood pressure, including hypertensive crisis with acute impairment of organ systems, has been reported on rare occasions in patients with and without a history of hypertension receiving 5-HT1 agonists, including MAXALT. In healthy young adult male and female patients who received maximal doses of MAXALT (10 mg every 2 hours for 3 doses), slight increases in blood pressure (approximately 2-3 mmHg) were observed. MAXALT is contraindicated in patients with uncontrolled hypertension [see Contraindications (4)].
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Rizatriptan in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Rizatriptan in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Rizatriptan in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Rizatriptan during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Rizatriptan with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Rizatriptan with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Rizatriptan with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Rizatriptan with respect to specific gender populations.
Race
There is no FDA guidance on the use of Rizatriptan with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Rizatriptan in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Rizatriptan in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Rizatriptan in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Rizatriptan in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Rizatriptan in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Rizatriptan in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Rizatriptan in the drug label.
Pharmacology
There is limited information regarding Rizatriptan Pharmacology in the drug label.
Mechanism of Action
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Rizatriptan in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Rizatriptan in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Rizatriptan in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Rizatriptan in the drug label.
How Supplied
Storage
There is limited information regarding Rizatriptan Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Rizatriptan |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Rizatriptan |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Rizatriptan in the drug label.
Precautions with Alcohol
- Alcohol-Rizatriptan interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Rizatriptan |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Rizatriptan |Label Name=Rizatriptan11.png
}}
{{#subobject:
|Label Page=Rizatriptan |Label Name=Rizatriptan11.png
}}