Ritonavir
{{DrugProjectFormSinglePage |authorTag=Stefano Giannoni [1] |genericName=Ritonavir |aOrAn=an |drugClass=antiretroviral agents |indicationType=treatment |indication=HIV-1 infection |hasBlackBoxWarning=Yes |adverseReactions=diarrhea, nausea, vomiting, abdominal pain, paresthesia, rash, and fatigue/asthenia |blackBoxWarningTitle= WARNING: DRUG-DRUG INTERACTIONS LEADING TO POTENTIALLY SERIOUS AND/OR LIFE THREATENING REACTIONS |blackBoxWarningBody=Co-administration with other drugs: Co-administration of NORVIR with several classes of drugs including sedative hypnotics, antiarrhythmics, or ergot alkaloid preparations may result in potentially serious and/or life-threatening adverse events due to possible effects of NORVIR on the hepatic metabolism of certain drugs. Review medications taken by patients prior to prescribing NORVIR or when prescribing other medications to patients already taking NORVIR |fdaLIADAdult=====HIV-1 infection====
- 600 mg twice daily
- Ritonavir should be started at no less than 300 mg twice daily and increased at 2 to 3 day intervals by 100 mg twice daily. The maximum dose of 600 mg twice daily.
|offLabelAdultGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Ritonavir in adult patients. |offLabelAdultNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Ritonavir in adult patients. |fdaLIADPed=====HIV-1 Infection====
- Children greater than 1 month is 350 to 400 mg per m2 twice daily.
- In combination with other antiretroviral agents.
- Not exceed 600 mg twice daily.
- Ritonavir should be started at 250 mg per m2 twice daily and increased at 2 to 3 day intervals by 50 mg per m2 twice daily.

|offLabelPedGuideSupport=There is limited information regarding Off-Label Guideline-Supported Use of Ritonavir in pediatric patients. |offLabelPedNoGuideSupport=There is limited information regarding Off-Label Non–Guideline-Supported Use of Ritonavir in pediatric patients. |contraindications=*When co-administering NORVIR with other protease inhibitors, see the full prescribing information for that protease inhibitor including contraindication information.
- NORVIR is contraindicated in patients with known hypersensitivity (e.g., toxic epidermal necrolysis (TEN) or Stevens-Johnson syndrome) to ritonavir or any of its ingredients.
- Co-administration of NORVIR with several classes of drugs (including sedative hypnotics, antiarrhythmics, or ergot alkaloid preparations) is contraindicated and may result in potentially serious and/or life-threatening adverse events due to possible effects of NORVIR on the hepatic metabolism of these drugs (see TABLE 2).
- Voriconazole and St. John’s Wort are exceptions in that co-administration of NORVIR and voriconazole results in a significant decrease in plasma concentrations of voriconazole, and co-administration of NORVIR with St. John’s Wort may result in decreased ritonavir plasma concentrations.

|warnings=When co-administering NORVIR with other protease inhibitors, see the full prescribing information for that protease inhibitor including important Warnings and Precautions.
5.1 Drug Interactions NORVIR is a CYP3A inhibitor. Initiating treatment with NORVIR in patients receiving medications metabolized by CYP3A or initiating medications metabolized by CYP3A in patients already maintained on NORVIR may result in increased plasma concentrations of concomitant medications. Higher plasma concentrations of concomitant medications can result in increased or prolonged therapeutic or adverse effects, potentially leading to severe, life-threatening or fatal events. The potential for drug-drug interactions must be considered prior to and during therapy with NORVIR. Review of other medications taken by patients and monitoring of patients for adverse effects is recommended during therapy with NORVIR.
See Table 2 for a listing of drugs that are contraindicated with NORVIR due to potentially life-threatening adverse events, significant drug interactions, or loss of virologic activity. Also, see Table 5 for a listing of drugs with established and other significant drug interactions [see Contraindications (4), Drug Interactions (7), and Clinical Pharmacology (12.3)].
5.2 Toxicity in Preterm Neonates NORVIR oral solution contains the excipients alcohol (43.2% v/v) and propylene glycol (26.57% w/v). When administered concomitantly with propylene glycol, ethanol competitively inhibits the metabolism of propylene glycol, which may lead to elevated concentrations. Preterm neonates may be at an increased risk of propylene glycol-associated adverse events due to diminished ability to metabolize propylene glycol, thereby leading to accumulation and potential adverse events. Postmarketing life-threatening cases of cardiac toxicity (including complete AV block, bradycardia, and cardiomyopathy), lactic acidosis, acute renal failure, CNS depression and respiratory complications leading to death have been reported, predominantly in preterm neonates receiving lopinavir/ritonavir oral solution which also contains the excipients alcohol and propylene glycol.
NORVIR oral solution should not be used in preterm neonates in the immediate postnatal period because of possible toxicities. However, if the benefit of using NORVIR oral solution to treat HIV infection in infants immediately after birth outweighs the potential risks, infants should be monitored closely for increases in serum osmolality and serum creatinine, and for toxicity related to NORVIR oral solution including: hyperosmolality, with or without lactic acidosis, renal toxicity, CNS depression (including stupor, coma, and apnea), seizures, hypotonia, cardiac arrhythmias and ECG changes, and hemolysis. Total amounts of alcohol and propylene glycol from all medicines that are to be given to infants should be taken into account in order to avoid toxicity from these excipients [see Dosage and Administration (2.2) and Overdosage (10)].
5.3 Hepatic Reactions Hepatic transaminase elevations exceeding 5 times the upper limit of normal, clinical hepatitis, and jaundice have occurred in patients receiving NORVIR alone or in combination with other antiretroviral drugs (see Table 4). There may be an increased risk for transaminase elevations in patients with underlying hepatitis B or C. Therefore, caution should be exercised when administering NORVIR to patients with pre-existing liver diseases, liver enzyme abnormalities, or hepatitis. Increased AST/ALT monitoring should be considered in these patients, especially during the first three months of NORVIR treatment [see Use in Specific Populations (8.6)].
There have been postmarketing reports of hepatic dysfunction, including some fatalities. These have generally occurred in patients taking multiple concomitant medications and/or with advanced AIDS.
5.4 Pancreatitis Pancreatitis has been observed in patients receiving NORVIR therapy, including those who developed hypertriglyceridemia. In some cases fatalities have been observed. Patients with advanced HIV disease may be at increased risk of elevated triglycerides and pancreatitis [see Warnings and Precautions (5.7)]. Pancreatitis should be considered if clinical symptoms (nausea, vomiting, abdominal pain) or abnormalities in laboratory values (such as increased serum lipase or amylase values) suggestive of pancreatitis should occur. Patients who exhibit these signs or symptoms should be evaluated and NORVIR therapy should be discontinued if a diagnosis of pancreatitis is made.
5.5 Allergic Reactions/Hypersensitivity Allergic reactions including urticaria, mild skin eruptions, bronchospasm, and angioedema have been reported. Cases of anaphylaxis, toxic epidermal necrolysis (TEN), and Stevens-Johnson syndrome have also been reported. Discontinue treatment if severe reactions develop.
5.6 PR Interval Prolongation Ritonavir prolongs the PR interval in some patients. Post marketing cases of second or third degree atrioventricular block have been reported in patients.
NORVIR should be used with caution in patients with underlying structural heart disease, preexisting conduction system abnormalities, ischemic heart disease, cardiomyopathies, as these patients may be at increased risk for developing cardiac conduction abnormalities.
The impact on the PR interval of co-administration of ritonavir with other drugs that prolong the PR interval (including calcium channel blockers, beta-adrenergic blockers, digoxin and atazanavir) has not been evaluated. As a result, co-administration of ritonavir with these drugs should be undertaken with caution, particularly with those drugs metabolized by CYP3A. Clinical monitoring is recommended [see Drug Interactions (7), and Clinical Pharmacology (12.3)].
5.7 Lipid Disorders Treatment with NORVIR therapy alone or in combination with saquinavir has resulted in substantial increases in the concentration of total cholesterol and triglycerides [see Adverse Reactions (6.1)]. Triglyceride and cholesterol testing should be performed prior to initiating NORVIR therapy and at periodic intervals during therapy. Lipid disorders should be managed as clinically appropriate, taking into account any potential drug-drug interactions with NORVIR and HMG CoA reductase inhibitors [see Contraindications (4)and Drug Interactions (7)].
5.8 Diabetes Mellitus/Hyperglycemia New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus, and hyperglycemia have been reported during postmarketing surveillance in HIV-infected patients receiving protease inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between protease inhibitor therapy and these events has not been established.
5.9 Immune Reconstitution Syndrome Immune reconstitution syndrome has been reported in HIV-infected patients treated with combination antiretroviral therapy, including NORVIR. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jiroveci pneumonia, or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves’ disease, polymyositis, and Guillain-Barré syndrome) have also been reported to occur in the setting of immune reconstitution, however, the time to onset is more variable, and can occur many months after initiation of treatment.
5.10 Fat Redistribution Redistribution/accumulation of body fat including central obesity, dorsocervical fat enlargement (buffalo hump), peripheral wasting, facial wasting, breast enlargement, and "cushingoid appearance" have been observed in patients receiving antiretroviral therapy. The mechanism and long-term consequences of these events are currently unknown. A causal relationship has not been established.
5.11 Patients with Hemophilia There have been reports of increased bleeding, including spontaneous skin hematomas and hemarthrosis, in patients with hemophilia type A and B treated with protease inhibitors. In some patients additional factor VIII was given. In more than half of the reported cases, treatment with protease inhibitors was continued or reintroduced. A causal relationship between protease inhibitor therapy and these events has not been established.
5.12 Resistance/Cross-resistance Varying degrees of cross-resistance among protease inhibitors have been observed. Continued administration of ritonavir 600 mg twice daily following loss of viral suppression may increase the likelihood of cross-resistance to other protease inhibitors [see Microbiology (12.4)].
5.13 Laboratory Tests Ritonavir has been shown to increase triglycerides, cholesterol, SGOT (AST), SGPT (ALT), GGT, CPK, and uric acid. Appropriate laboratory testing should be performed prior to initiating NORVIR therapy and at periodic intervals or if any clinical signs or symptoms occur during therapy. |clinicalTrials=When co-administering NORVIR with other protease inhibitors, see the full prescribing information for that protease inhibitor including adverse reactions.
6.1 Adult Clinical Trial Experience Because clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of NORVIR alone and in combination with other antiretroviral agents was studied in 1,755 adult patients. Table 3 lists treatment-emergent Adverse Reactions (with possible or probable relationship to study drug) occurring in greater than or equal to 1% of adult patients receiving NORVIR in combined Phase II/IV studies.
The most frequently reported adverse drug reactions among patients receiving NORVIR alone or in combination with other antiretroviral drugs were gastrointestinal (including diarrhea, nausea, vomiting, abdominal pain (upper and lower)), neurological disturbances (including paresthesia and oral paresthesia), rash, and fatigue/asthenia.


Pediatric Clinical Trial Experience NORVIR has been studied in 265 pediatric patients greater than 1 month to 21 years of age. The adverse event profile observed during pediatric clinical trials was similar to that for adult patients.
Vomiting, diarrhea, and skin rash/allergy were the only drug-related clinical adverse events of moderate to severe intensity observed in greater than or equal to 2% of pediatric patients enrolled in NORVIR clinical trials.
Laboratory Abnormalities
The following Grade 3-4 laboratory abnormalities occurred in greater than 3% of pediatric patients who received treatment with NORVIR either alone or in combination with reverse transcriptase inhibitors: neutropenia (9%), hyperamylasemia (7%), thrombocytopenia (5%), anemia (4%), and elevated AST (3%). |postmarketing=The following adverse events (not previously mentioned in the labeling) have been reported during post-marketing use of NORVIR. Because these reactions are reported voluntarily from a population of unknown size, it is not possible to reliably estimate their frequency or establish a causal relationship to NORVIR exposure.
Body as a Whole
Dehydration, usually associated with gastrointestinal symptoms, and sometimes resulting in hypotension, syncope, or renal insufficiency has been reported. Syncope, orthostatic hypotension, and renal insufficiency have also been reported without known dehydration.
Co-administration of ritonavir with ergotamine or dihydroergotamine has been associated with acute ergot toxicity characterized by vasospasm and ischemia of the extremities and other tissues including the central nervous system.
Cardiovascular System
First-degree AV block, second-degree AV block, third-degree AV block, right bundle branch block have been reported [see Warnings and Precautions (5.6)].
Cardiac and neurologic events have been reported when ritonavir has been co-administered with disopyramide, mexiletine, nefazodone, fluoxetine, and beta blockers. The possibility of drug interaction cannot be excluded.
Endocrine System
Cushing's syndrome and adrenal suppression have been reported when ritonavir has been co-administered with fluticasone propionate or budesonide.
Nervous System
There have been postmarketing reports of seizure. Also, see Cardiovascular System.
Skin and subcutaneous tissue disorders
Toxic epidermal necrolysis (TEN) has been reported. |drugInteractions=When co-administering NORVIR with other protease inhibitors (atazanavir, darunavir, fosamprenavir, saquinavir, and tipranavir), see the full prescribing information for that protease inhibitor including important information for drug interactions.
7.1 Potential for NORVIR to Affect Other Drugs Ritonavir has been found to be an inhibitor of cytochrome P450 3A (CYP3A) and may increase plasma concentrations of agents that are primarily metabolized by CYP3A. Agents that are extensively metabolized by CYP3A and have high first pass metabolism appear to be the most susceptible to large increases in AUC (greater than 3-fold) when co-administered with ritonavir. Thus, co-administration of NORVIR with drugs highly dependent on CYP3A for clearance and for which elevated plasma concentrations are associated with serious and/or life-threatening events is contraindicated. Co-administration with other CYP3A substrates may require a dose adjustment or additional monitoring as shown in Table 5.
Ritonavir also inhibits CYP2D6 to a lesser extent. Co-administration of substrates of CYP2D6 with ritonavir could result in increases (up to 2-fold) in the AUC of the other agent, possibly requiring a proportional dosage reduction. Ritonavir also appears to induce CYP3A, CYP1A2, CYP2C9, CYP2C19, and CYP2B6 as well as other enzymes, including glucuronosyl transferase.
7.2 Established and Other Potentially Significant Drug Interactions Table 5 provides a list of established or potentially clinically significant drug interactions. Alteration in dose or regimen may be recommended based on drug interaction studies or predicted interaction [see Clinical Pharmacology (12.3) for magnitude of interaction].

|FDAPregCat=B |useInPregnancyFDA=Human Data: There are no adequate and well-controlled studies in pregnant women. NORVIR should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal Data No treatment related malformations were observed when ritonavir was administered to pregnant rats or rabbits. Developmental toxicity observed in rats (early resorptions, decreased fetal body weight and ossification delays and developmental variations) occurred at a maternally toxic dosage at an exposure equivalent to approximately 30% of that achieved with the proposed therapeutic dose. A slight increase in the incidence of cryptorchidism was also noted in rats at an exposure approximately 22% of that achieved with the proposed therapeutic dose.
Developmental toxicity observed in rabbits (resorptions, decreased litter size and decreased fetal weights) also occurred at a maternally toxic dosage equivalent to 1.8 times the proposed therapeutic dose based on a body surface area conversion factor. |AUSPregCat=B3 |useInNursing=The Centers for Disease Control and Prevention recommend that HIV-infected mothers not breastfeed their infants to avoid risking postnatal transmission of HIV. It is not known whether ritonavir is secreted in human milk. Because of both the potential for HIV transmission and the potential for serious adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving NORVIR. |useInPed=In HIV-infected patients age greater than 1 month to 21 years, the antiviral activity and adverse event profile seen during clinical trials and through postmarketing experience were similar to that for adult patients. |useInGeri=Clinical studies of NORVIR did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy. |useInHepaticImpair=No dose adjustment of ritonavir is necessary for patients with either mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. No pharmacokinetic or safety data are available regarding the use of ritonavir in subjects with severe hepatic impairment (Child-Pugh Class C), therefore, ritonavir is not recommended for use in patients with severe hepatic impairment [see Warnings and Precautions (5.3), Clinical Pharmacology (12.3)]. |overdose=10.1 Acute Overdosage - Human Overdose Experience Human experience of acute overdose with NORVIR is limited. One patient in clinical trials took NORVIR 1500 mg per day for two days. The patient reported paresthesias which resolved after the dose was decreased. A post-marketing case of renal failure with eosinophilia has been reported with ritonavir overdose.
The approximate lethal dose was found to be greater than 20 times the related human dose in rats and 10 times the related human dose in mice.
10.2 Management of Overdosage NORVIR oral solution contains 43.2% (v/v) alcohol and 26.57% (w/v) propylene glycol. Ingestion of the product over the recommended dose by a young child could result in significant toxicity and could potentially be lethal.
Treatment of overdose with NORVIR consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient. There is no specific antidote for overdose with NORVIR. If indicated, elimination of unabsorbed drug should be achieved by gastric lavage; usual precautions should be observed to maintain the airway. Administration of activated charcoal may also be used to aid in removal of unabsorbed drug. Since ritonavir is extensively metabolized by the liver and is highly protein bound, dialysis is unlikely to be beneficial in significant removal of the drug. However, dialysis can remove both alcohol and propylene glycol in the case of overdose with ritonavir oral solution. A Certified Poison Control Center should be consulted for up-to-date information on the management of overdose with NORVIR |drugBox={{Drugbox | Verifiedfields = changed | verifiedrevid = 477172835 | IUPAC_name = 1,3-thiazol-5-ylmethyl N-[(2S,3S,5S)-3-hydroxy-5-[(2S)-3-methyl-2-{[methyl({[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl})carbamoyl]amino}butanamido]-1,6-diphenylhexan-2-yl]carbamate | image = Ritonavir chemical structure 2.png
| tradename = Norvir | Drugs.com = Monograph | MedlinePlus = a696029 | pregnancy_AU = B3 | pregnancy_US = B | legal_AU = S4 | legal_CA = Rx-only | legal_UK = POM | legal_US = Rx-only | routes_of_administration = oral
| bioavailability = | protein_bound = 98-99% | metabolism = Hepatic | elimination_half-life = 3-5 hours | excretion = mostly fecal
| CAS_number_Ref =
| CAS_number = 155213-67-5
| ATC_prefix = J05
| ATC_suffix = AE03
| ATC_supplemental =
| PubChem = 392622
| DrugBank_Ref =
| DrugBank = DB00503
| ChemSpiderID_Ref =
| ChemSpiderID = 347980
| UNII_Ref =
| UNII = O3J8G9O825
| KEGG_Ref =
| KEGG = D00427
| ChEBI_Ref =
| ChEBI = 45409
| ChEMBL_Ref =
| ChEMBL = 163
| NIAID_ChemDB = 028478
| C=37 | H=48 | N=6 | O=5 | S=2
| molecular_weight = 720.946 g/mol
| smiles = CC(C)c4nc(CN(C)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccccc1)C[C@H](O)[C@H](Cc2ccccc2)NC(=O)OCc3cncs3)cs4
| InChI = 1/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1
| InChIKey = NCDNCNXCDXHOMX-XGKFQTDJBG
| StdInChI_Ref =
| StdInChI = 1S/C37H48N6O5S2/c1-24(2)33(42-36(46)43(5)20-29-22-49-35(40-29)25(3)4)34(45)39-28(16-26-12-8-6-9-13-26)18-32(44)31(17-27-14-10-7-11-15-27)41-37(47)48-21-30-19-38-23-50-30/h6-15,19,22-25,28,31-33,44H,16-18,20-21H2,1-5H3,(H,39,45)(H,41,47)(H,42,46)/t28-,31-,32-,33-/m0/s1
| StdInChIKey_Ref =
| StdInChIKey = NCDNCNXCDXHOMX-XGKFQTDJSA-N
}}
|mechAction=Ritonavir is a peptidomimetic inhibitor of the HIV-1 protease. Inhibition of HIV protease renders the enzyme incapable of processing the gag-pol polyprotein precursor which leads to production of non-infectious immature HIV particles.
|structure=Ritonavir is chemically designated as 10-Hydroxy-2-methyl-5-(1-methylethyl)-1- [2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-2,4,7,12- tetraazatridecan-13-oic acid, 5-thiazolylmethyl ester, [5S-(5R*,8R*,10R*,11R*)]. Its molecular formula is C37H48N6O5S2, and its molecular weight is 720.95. Ritonavir has the following structural formula:
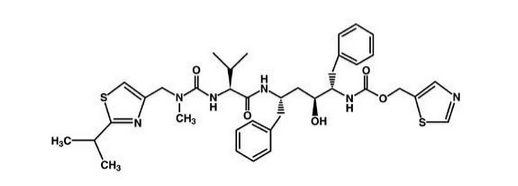
|PK=The pharmacokinetics of ritonavir have been studied in healthy volunteers and HIV-infected patients (CD4 greater than or equal to 50 cells per μL). See Table 6 for ritonavir pharmacokinetic characteristics.
Absorption
The absolute bioavailability of ritonavir has not been determined. After a 600 mg dose of oral solution, peak concentrations of ritonavir were achieved approximately 2 hours and 4 hours after dosing under fasting and non-fasting (514 KCal; 9% fat, 12% protein, and 79% carbohydrate) conditions, respectively.
NORVIR tablets are not bioequivalent to NORVIR capsules. Under moderate fat conditions (857 kcal; 31% fat, 13% protein, 56% carbohydrates), when a single 100 mg NORVIR dose was administered as a tablet compared with a capsule, AUC(0- ∞) met equivalence criteria but mean Cmax was increased by 26% (92.8% confidence intervals: ↑15 -↑39%).
No information is available comparing NORVIR tablets to NORVIR capsules under fasting conditions.
Effect of Food on Oral Absorption
When the oral solution was given under non-fasting conditions, peak ritonavir concentrations decreased 23% and the extent of absorption decreased 7% relative to fasting conditions. Dilution of the oral solution, within one hour of administration, with 240 mL of chocolate milk, Advera® or Ensure® did not significantly affect the extent and rate of ritonavir absorption. Administration of a single 600 mg dose oral solution under non-fasting conditions yielded mean ± SD areas under the plasma concentration-time curve (AUCs) of 129.0 ± 39.3 mg•h per mL.
A food effect is observed for NORVIR tablets. Food decreased the bioavailability of the ritonavir tablets when a single 100 mg dose of NORVIR was administered. Under high fat conditions (907 kcal; 52% fat, 15% protein, 33% carbohydrates), a 23% decrease in mean AUC(0-∞) [90% confidence intervals: ↓30%-↓15%], and a 23% decrease in mean Cmax [90% confidence intervals: ↓34%-↓11%]) was observed relative to fasting conditions. Under moderate fat conditions, a 21% decrease in mean AUC(0-∞) [90% confidence intervals: ↓28%-↓13%], and a 22% decrease in mean Cmax [90% confidence intervals: ↓33%-↓9%]) was observed relative to fasting conditions.
However, the type of meal administered did not change ritonavir tablet bioavailability when high fat was compared to moderate fat meals.
Metabolism
Nearly all of the plasma radioactivity after a single oral 600 mg dose of 14C-ritonavir oral solution (n = 5) was attributed to unchanged ritonavir. Five ritonavir metabolites have been identified in human urine and feces. The isopropylthiazole oxidation metabolite (M-2) is the major metabolite and has antiviral activity similar to that of parent drug; however, the concentrations of this metabolite in plasma are low. In vitro studies utilizing human liver microsomes have demonstrated that cytochrome P450 3A (CYP3A) is the major isoform involved in ritonavir metabolism, although CYP2D6 also contributes to the formation of M–2.
Elimination
In a study of five subjects receiving a 600 mg dose of 14C-ritonavir oral solution, 11.3 ± 2.8% of the dose was excreted into the urine, with 3.5 ± 1.8% of the dose excreted as unchanged parent drug. In that study, 86.4 ± 2.9% of the dose was excreted in the feces with 33.8 ± 10.8% of the dose excreted as unchanged parent drug. Upon multiple dosing, ritonavir accumulation is less than predicted from a single dose possibly due to a time and dose-related increase in clearance.

Effects on Electrocardiogram
QTcF interval was evaluated in a randomized, placebo and active (moxifloxacin 400 mg once-daily) controlled crossover study in 45 healthy adults, with 10 measurements over 12 hours on Day 3. The maximum mean (95% upper confidence bound) time-matched difference in QTcF from placebo after baseline correction was 5.5 (7.6) milliseconds (msec) for 400 mg twice-daily ritonavir. Ritonavir 400 mg twice daily resulted in Day 3 ritonavir exposure that was approximately 1.5 fold higher than observed with ritonavir 600 mg twice-daily dose at steady state.
PR interval prolongation was also noted in subjects receiving ritonavir in the same study on Day 3. The maximum mean (95% confidence interval) difference from placebo in the PR interval after baseline correction was 22 (25) msec for 400 mg twice-daily ritonavir [see Warnings and Precautions (5.6)].
Special Populations
Gender, Race and Age
No age-related pharmacokinetic differences have been observed in adult patients (18 to 63 years). Ritonavir pharmacokinetics have not been studied in older patients.
A study of ritonavir pharmacokinetics in healthy males and females showed no statistically significant differences in the pharmacokinetics of ritonavir. Pharmacokinetic differences due to race have not been identified.
Pediatric Patients
Steady-state pharmacokinetics were evaluated in 37 HIV-infected patients ages 2 to 14 years receiving doses ranging from 250 mg per m2 twice-daily to 400 mg per m2 twice-daily in PACTG Study 310, and in 41 HIV-infected patients ages 1 month to 2 years at doses of 350 and 450 mg per m2 twice-daily in PACTG Study 345. Across dose groups, ritonavir steady-state oral clearance (CL/F/m2) was approximately 1.5 to 1.7 times faster in pediatric patients than in adult subjects. Ritonavir concentrations obtained after 350 to 400 mg per m2 twice-daily in pediatric patients greater than 2 years were comparable to those obtained in adults receiving 600 mg (approximately 330 mg per m2) twice-daily. The following observations were seen regarding ritonavir concentrations after administration with 350 or 450 mg per m2 twice-daily in children less than 2 years of age. Higher ritonavir exposures were not evident with 450 mg per m2 twice-daily compared to the 350 mg per m2 twice-daily. Ritonavir trough concentrations were somewhat lower than those obtained in adults receiving 600 mg twice-daily. The area under the ritonavir plasma concentration time curve and trough concentrations obtained after administration with 350 or 450 mg per m2 twice-daily in children less than 2 years were approximately 16% and 60% lower, respectively, than that obtained in adults receiving 600 mg twice daily.
Renal Impairment
Ritonavir pharmacokinetics have not been studied in patients with renal impairment, however, since renal clearance is negligible, a decrease in total body clearance is not expected in patients with renal impairment.
Hepatic Impairment
Dose-normalized steady-state ritonavir concentrations in subjects with mild hepatic impairment (400 mg twice-daily, n = 6) were similar to those in control subjects dosed with 500 mg twice-daily. Dose-normalized steady-state ritonavir exposures in subjects with moderate hepatic impairment (400 mg twice-daily, n= 6) were about 40% lower than those in subjects with normal hepatic function (500 mg twice-daily, n = 6). Protein binding of ritonavir was not statistically significantly affected by mild or moderately impaired hepatic function. No dose adjustment is recommended in patients with mild or moderate hepatic impairment. However, health care providers should be aware of the potential for lower ritonavir concentrations in patients with moderate hepatic impairment and should monitor patient response carefully. Ritonavir has not been studied in patients with severe hepatic impairment.
Drug Interactions
[see also Contraindications (4), Warnings and Precautions (5.1), and Drug Interactions (7)]
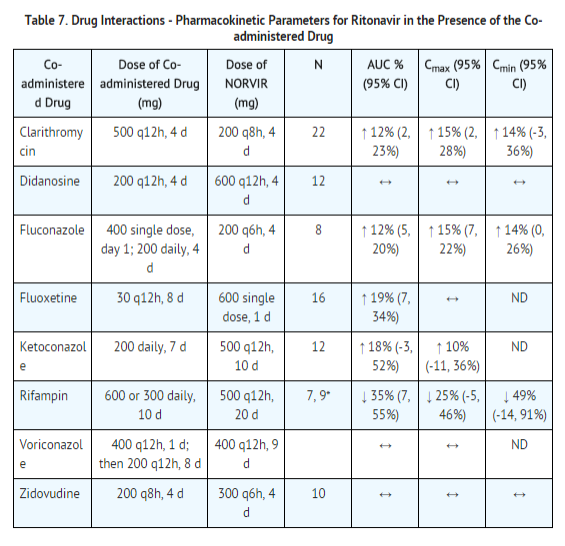
Table 7 and Table 8 summarize the effects on AUC and Cmax, with 95% confidence intervals (95% CI), of co-administration of ritonavir with a variety of drugs. For information about clinical recommendations see Table 5 in Drug Interactions (7).

|nonClinToxic= Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Carcinogenicity studies in mice and rats have been carried out on ritonavir. In male mice, at levels of 50, 100 or 200 mg per kg per day, there was a dose dependent increase in the incidence of both adenomas and combined adenomas and carcinomas in the liver. Based on AUC measurements, the exposure at the high dose was approximately 0.3-fold for males that of the exposure in humans with the recommended therapeutic dose (600 mg twice-daily). There were no carcinogenic effects seen in females at the dosages tested. The exposure at the high dose was approximately 0.6-fold for the females that of the exposure in humans. In rats dosed at levels of 7, 15 or 30 mg per kg per day there were no carcinogenic effects. In this study, the exposure at the high dose was approximately 6% that of the exposure in humans with the recommended therapeutic dose. Based on the exposures achieved in the animal studies, the significance of the observed effects is not known.
Mutagenesis
However, ritonavir was found to be negative for mutagenic or clastogenic activity in a battery of in in vitro and in vivo assays including the Ames bacterial reverse mutation assay using S. typhimurium and E. coli, the mouse lymphoma assay, the mouse micronucleus test and chromosomal aberration assays in human lymphocytes.
Impairment of Fertility
Ritonavir produced no effects on fertility in rats at drug exposures approximately 40% (male) and 60% (female) of that achieved with the proposed therapeutic dose. Higher dosages were not feasible due to hepatic toxicity. |clinicalStudies=The activity of NORVIR as monotherapy or in combination with nucleoside reverse transcriptase inhibitors has been evaluated in 1446 patients enrolled in two double-blind, randomized trials.
14.1 Advanced Patients with Prior Antiretroviral Therapy Study 247 was a randomized, double-blind trial (with open-label follow-up) conducted in HIV-infected patients with at least nine months of prior antiretroviral therapy and baseline CD4 cell counts less than or equal to 100 cells per μL. NORVIR 600 mg twice-daily or placebo was added to each patient's baseline antiretroviral therapy regimen, which could have consisted of up to two approved antiretroviral agents. The study accrued 1,090 patients, with mean baseline CD4 cell count at study entry of 32 cells per μL. After the clinical benefit of NORVIR therapy was demonstrated, all patients were eligible to switch to open-label NORVIR for the duration of the follow-up period. Median duration of double-blind therapy with NORVIR and placebo was 6 months. The median duration of follow-up through the end of the open-label phase was 13.5 months for patients randomized to NORVIR and 14 months for patients randomized to placebo.
The cumulative incidence of clinical disease progression or death during the double-blind phase of Study 247 was 26% for patients initially randomized to NORVIR compared to 42% for patients initially randomized to placebo. This difference in rates was statistically significant.
Cumulative mortality through the end of the open-label follow-up phase for patients enrolled in Study 247 was 18% (99/543) for patients initially randomized to NORVIR compared to 26% (142/547) for patients initially randomized to placebo. This difference in rates was statistically significant. However, since the analysis at the end of the open-label phase includes patients in the placebo arm who were switched from placebo to NORVIR therapy, the survival benefit of NORVIR cannot be precisely estimated.
During the double-blind phase of Study 247, CD4 cell counts increases from baseline for patients randomized to NORVIR at Week 2 and Week 4 were observed. From Week 4 and through Week 24, mean CD4 cell counts for patients randomized to NORVIR appeared to plateau. In contrast, there was no apparent change in mean CD4 cell counts for patients randomized to placebo at any visit between baseline and Week 24 of the double-blind phase of Study 247.
14.2 Patients without Prior Antiretroviral Therapy In Study 245, 356 antiretroviral-naive HIV-infected patients (mean baseline CD4 = 364 cells per μL) were randomized to receive either NORVIR 600 mg twice-daily, zidovudine 200 mg three-times-daily, or a combination of these drugs.
During the double-blind phase of study 245, greater mean CD4 cell count increases were observed from baseline to Week 12 in the NORVIR-containing arms compared to the zidovudine arms. Mean CD4 cell count changes subsequently appeared to plateau through Week 24 in the NORVIR arm, whereas mean CD4 cell counts gradually diminished through Week 24 in the zidovudine and NORVIR plus zidovudine arms.
Greater mean reductions in plasma HIV-1 RNA levels were observed from baseline to Week 2 for the NORVIR-containing arms compared to the zidovudine arm. After Week 2 and through Week 24, mean plasma HIV-1 RNA levels either remained stable in the NORVIR and zidovudine arms or gradually rebounded toward baseline in the NORVIR plus zidovudine arm. |howSupplied=====NORVIR Tablets====
- 100 mg Ritonavir
NORVIR (ritonavir) tablets are white film-coated ovaloid tablets debossed with the "a" logo and the code NK.
NORVIR Oral Solution
- 80 mg per mL Ritonavir
NORVIR (ritonavir) oral solution is an orange-colored liquid, supplied in amber-colored, multi-dose bottles containing 600 mg ritonavir per 7.5 mL marked dosage cup (80 mg per mL).
240 mL bottles (NDC 0074-1940-63).
Bottles of 30 tablets each (NDC 0074-3333-30). |storage=====NORVIR Tablets==== Store at or below 30°C (86°F). Exposure to temperatures up to 50°C (122°F) for seven days permitted. Dispense in original container or USP equivalent tight container (60 mL or less). For patient use: exposure of this product to high humidity outside the original or USP equivalent tight container (60 mL or less) for longer than 2 weeks is not recommended.
NORVIR Oral Solution
Store NORVIR oral solution at room temperature 20°-25°C (68°-77°F). Do not refrigerate. Shake well before each use. Use by product expiration date.
|packLabel=


|fdaPatientInfo=Patients or parents of patients should be informed that:
General Information
They should pay special attention to accurate administration of their dose to minimize the risk of accidental overdose or underdose of NORVIR.
They should inform their healthcare provider if their children’s weight changes in order to make sure that the child’s NORVIR dose is the correct one.
Take NORVIR with meals.
For adult patients taking NORVIR tablets, the maximum dose of 600 mg twice daily by mouth with meals should not be exceeded.
Patients should remain under the care of a physician while using NORVIR. Patients should be advised to take NORVIR and other concomitant antiretroviral therapy every day as prescribed. NORVIR must always be used in combination with other antiretroviral drugs. Patients should not alter the dose or discontinue therapy without consulting with their doctor. If a dose of NORVIR is missed patients should take the dose as soon as possible and then return to their normal schedule. However, if a dose is skipped the patient should not double the next dose.
NORVIR is not a cure for HIV-1 infection and patients may continue to experience illnesses associated with HIV-1 infection, including opportunistic infections. Patients should remain under the care of a physician when using NORVIR.
Patients should be advised to avoid doing things that can spread HIV-1 infection to others.
Do not share needles or other injection equipment. Do not share personal items that can have blood or body fluids on them, like toothbrushes and razor blades. Do not have any kind of sex without protection. Always practice safe sex by using a latex or polyurethane condom to lower the chance of sexual contact with semen, vaginal secretions, or blood. Do not breastfeed. We do not know if NORVIR can be passed to the baby through breast milk and whether it could harm the baby. Also, mothers with HIV-1 should not breastfeed because HIV-1 can be passed to the baby in the breast milk. Sustained decreases in plasma HIV-1 RNA have been associated with a reduced risk of progression to AIDS and death.
Drug Interactions
NORVIR may interact with some drugs; therefore, patients should be advised to report to their doctor the use of any other prescription, non-prescription medication or herbal products, particularly St. John's Wort.
If they are receiving estrogen-based hormonal contraceptives, additional or alternate contraceptive measures should be used during therapy with NORVIR.
Potential Adverse Effects
Pre-existing liver disease including Hepatitis B or C can worsen with use of NORVIR. This can be seen as worsening of transaminase elevations or hepatic decompensation. Patients should be advised that their liver function tests will need to be monitored closely especially during the first several months of NORVIR treatment and that they should notify their healthcare provider if they develop the signs and symptoms of worsening liver disease including loss of appetite, abdominal pain, jaundice, and itchy skin.
Pancreatitis, including some fatalities, has been observed in patients receiving NORVIR therapy. Your patients should let you know of signs and symptoms (nausea, vomiting, and abdominal pain) that might be suggestive of pancreatitis.
Skin rashes ranging in severity from mild to Stevens-Johnson syndrome have been reported in patients receiving NORVIR. Patients should be advised to contact their healthcare provider if they develop a rash while taking NORVIR. The healthcare provider will determine if treatment should be continued or an alternative antiretroviral regimen used.
NORVIR may produce changes in the electrocardiogram (e.g., PR prolongation). Patients should consult their physician if they experience symptoms such as dizziness, lightheadedness, abnormal heart rhythm or loss of consciousness.
Treatment with NORVIR therapy can result in substantial increases in the concentration of total cholesterol and triglycerides.
New onset of diabetes or exacerbation of pre-existing diabetes mellitus, and hyperglycemia have been reported. Patients should be advised to notify their healthcare provider if they develop the signs and symptoms of diabetes mellitus including frequent urination, excessive thirst, extreme hunger or unusual weight loss and/or an increased blood sugar while on NORVIR as they may require a change in their diabetes treatment or new treatment.
Immune reconstitution syndrome has been reported in HIV-infected patients treated with combination antiretroviral therapy, including NORVIR.
Redistribution or accumulation of body fat may occur in patients receiving antiretroviral therapy and that the cause and long term health effects of these conditions are not known at this time.
Patients with hemophilia may experience increased bleeding when treated with protease inhibitors such as NORVIR.
If they are receiving avanafil, sildenafil, tadalafil, or vardenafil for the treatment of erectile dysfunction, they may be at an increased risk of associated adverse reactions including hypotension, visual changes, and sustained erection, and should promptly report any symptoms to their doctor. They should seek medical assistance immediately if they develop a sustained penile erection lasting more than 4 hours while taking NORVIR and a PDE 5 Inhibitor such as Stendra®, Viagra®, Cialis® or Levitra®. If they are currently using or planning to use avanafil or tadalafil (for the treatment of pulmonary arterial hypertension) they should ask their doctor about potential adverse reactions these medications may cause when taken with NORVIR. The doctor may choose not to keep them on avanafil, or may adjust the dose of tadalafil while initiating treatment with NORVIR. Concomitant use of Revatio® (sildenafil) with NORVIR is contraindicated in patients with pulmonary arterial hypertension (PAH).
Continued NORVIR therapy at a dose of 600 mg twice daily following loss of viral suppression may increase the likelihood of cross-resistance to other protease inhibitors. |alcohol=Alcohol-Ritonavir interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. |brandNames=*Norvir }} {{#subobject:
|Label Page=Ritonavir |Label Name=Ritonavir Package.png
}}
{{#subobject:
|Label Page=Ritonavir |Label Name=Ritonavir package 2.png
}}
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [3]
Overview
Ritonavir, with trade name Norvir (AbbVie, Inc.), is an antiretroviral drug from the protease inhibitor class used to treat HIV infection and AIDS.
Ritonavir is frequently prescribed with HAART, not for its antiviral action, but as it inhibits the same host enzyme that metabolizes other protease inhibitors. This inhibition leads to higher plasma concentrations of these latter drugs, allowing the clinician to lower their dose and frequency and improving their clinical efficacy.
Category
Protease inhibitor
US Brand Names
NORVIR®
FDA Package Insert
Description | Clinical Pharmacology | Microbiology | Indications and Usage | Contraindications | Warnings and Precautions | Adverse Reactions | Drug Interactions | Overdosage | Clinical Studies | Dosage and Administration | How Supplied | Labels and Packages
Mechanism of Action
Ritonavir was originally developed as an inhibitor of HIV protease. It is one of the most complex inhibitors. It is now rarely used for its own antiviral activity, but remains widely used as a booster of other protease inhibitors. More specifically, ritonavir is used to inhibit a particular liver enzyme that normally metabolizes protease inhibitors, cytochrome P450-3A4 (CYP3A4).[1] The drug's molecular structure inhibits CYP3A4, so a low dose can be used to enhance other protease inhibitors. This discovery, which has drastically reduced the adverse effects and improved the efficacy of protease inhibitors and HAART, was first communicated in an article published in the journal AIDS in 1997 by researchers at the University of Liverpool.[2] This effect does come with a price: it also affects the efficacy of numerous other medications, making it difficult to know how to administer them concurrently. In addition it can cause a large number of side-effects on its own.
References
- ↑ Zeldin RK, Petruschke RA (2004). "Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients". Journal of Antimicrobial Chemotherapy. 53 (1): 4–9. doi:10.1093/jac/dkh029. PMID 14657084.
- ↑ Merry, Concepta; Barry, Michael G.; Mulcahy, Fiona; Ryan, Mairin; Heavey, Jane; Tjia, John F.; Gibbons, Sara E.; Breckenridge, Alasdair M.; Back, David J. (1997). "Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients". AIDS. 11 (4): F29–F33. doi:10.1097/00002030-199704000-00001. PMID 9084785.