Rimonabant
 | |
| Clinical data | |
|---|---|
| [[Regulation of therapeutic goods |Template:Engvar data]] | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Undetermined |
| Protein binding | Nearly 100% |
| Metabolism | Hepatic, CYP3A4 involved |
| Elimination half-life | Variable: 6 to 9 days with normal BMI 16 days if BMI >30 |
| Excretion | Fecal (86%) and renal (3%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
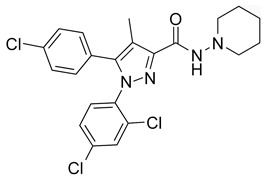
| Formula | C22H21Cl3N4O |
| Molar mass | 463.79 g/mol |
|
WikiDoc Resources for Rimonabant |
|
Articles |
|---|
|
Most recent articles on Rimonabant |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Rimonabant at Clinical Trials.gov Clinical Trials on Rimonabant at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Rimonabant
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Rimonabant Discussion groups on Rimonabant Patient Handouts on Rimonabant Directions to Hospitals Treating Rimonabant Risk calculators and risk factors for Rimonabant
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Rimonabant |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Rimonabant (also known as SR141716, Acomplia, Riobant, Slimona, Rimoslim, and Zimulti)[1] is an anorectic anti-obesity drug. It is a CB1 cannabinoid receptor antagonist. Its main avenue of effect is reduction in appetite.
Rimonabant is the first selective CB1 receptor blocker to be approved for use anywhere in the world. In Europe, it is indicated for use in conjunction with diet and exercise for patients with a body mass index greater than 30 kg/m², or patients wih a BMI greater than 27 kg/m² with associated risk factors, such as type 2 diabetes or dyslipidaemia. In the UK, it has been available since the end of July 2006. As of 2007, the drug was available in 38 countries.
Approval
Despite the FDA issuing an approvable letter in February 2006 for the obesity indication and a non-approvable letter for smoking cessation, the drug did not enter the market in the United States in 2006. The French pharma firm Sanofi-Aventis disclosed that a complete response to the FDA's approvable letter was submitted on October 26, 2006, triggering a Class I (two-month) or Class II (six-month) review process. On June 13, 2007, FDA's Endocrine and Metabolic Drugs Advisory Committee (EMDAC) concluded that the French manufacturer Sanofi-Aventis failed to demonstrate the safety of rimonabant and voted against recommending the anti-obesity treatment for approval.[2] Subsequently, Sanofi-Aventis announced that it was withdrawing the new drug application (NDA) for rimonabant and that it would resubmit an application at some point in the future.
On 21 June 2006, the European Commission approved the sale of rimonabant in the then 25-member European Union. Sanofi announced that the first country in which Acomplia will be sold is the United Kingdom. Sales began in July 2006. Sanofi also announced that it projects that the drug will be sold shortly thereafter in Denmark, Ireland, Germany, Finland and Norway. It is expected in Belgium[3] and Sweden in 2007. Ordinary obesity will, according to official medical recommendations, not be enough to acquire the prescription in Sweden; there are additional requirements concerning abnormal blood lipid levels.[4]
The EU's approval was not a blanket approval, nor did it approve Acomplia for non-obesity related problems such as smoking cessation, although off-label use of the drug is still possible. The approval is in combination with diet and exercise for the treatment of obese patients (BMI greater than or equal to 30), or overweight patients (BMI greater than 27) with associated risk factors, such as type 2 diabetes or dyslipidaemia.
Side effects
Shortly after market introduction, press reports and independent studies suggest that side effects occur stronger and more commonly than shown by the manufacturer in their clinical studies. Reports of severe depression are frequent. This is deemed to result from the drug being active in the central nervous system, an area of human physiology so complex that drug effects are highly difficult to determine reliably.[5]
Because the drug has the opposite effects of cannabinoid receptor agonists such as tetrahydrocannabinol, which is neuroprotective against excitotoxicity,[6] it can be theorized that Rimonabant promotes the development of neurodegenerative diseases of the central nervous system such as Multiple sclerosis, Alzheimer's disease, Amyotrophic lateral sclerosis (ALS), Parkinson's disease, and Huntington's disease in persons who are susceptible.[7] The reported development of previously clinically silent multiple sclerosis in one patient taking Rimonabant suggests that any patients with an underlying neurological condition should not take Rimonabant, given the neuroprotective role of the endocannabinoid system in many experimental paradigms of neurological disease.
On 15 June 2007 the BBC News reported [8] that a committee advising the US FDA has voted not to recommend the drug's approval because of concerns over suicidality, depression and other related side effects associated with use of the drug.
Similarly, in October 2008 marketing and new prescriptions of Acomplia (Rimonabant, sanofi-aventis) were suspended by the European Medicines Agency (EMEA). While more than 700,000 patients have taken the drug in over 18 countries, the EMEA sited the significant amount of psychiatric side effects and limited clinical effectiveness seen during post-marketing experience as reasons for the recommendation. Sanofi-aventis released a statement indicating both its compliance with the temporary suspension and its commitment to providing more evidence to the EMEA in support of the drug.[9]
Smoking cessation
Rimonabant may also be found to be effective in assisting some smokers to quit smoking. Sanofi-Aventis is currently conducting studies to determine the possible value of rimonabant in smoking-cessation therapy. The Studies with Rimonabant and Tobacco Use (STRATUS) Program involves more than 6,000 subjects. STRATUS is designed to explore two smoking-related therapies: first, to use rimonabant directly to aid in smoking cessation; second, to help prevent weight gain in former smokers. Initial results apparently suggest that rimonabant is effective for both uses. However, the FDA has explicitly stated to Sanofi-Aventis that without additional studies rimonabant cannot be approved in the United States for smoking cessation therapy. According to Cochrane review in 2007 Rimonabant "may increase the odds of quitting approximately 1(1/2)-fold"[10].
Addiction
Rimonabant reduced resumption of cocaine-seeking responses triggered by two of the three most common triggers of relapse in humans, priming and cues. It may also reduce ethanol and opiate seeking behavior[11].
Memory
Tetrahydrocannabinol is known to impair short-term memory. It was therefore hypothesised that Rimonabant may improve short-term memory. Indeed in animal studies it significantly improved the performance of rats to encode information in the short-term memory[12].
Blockade of Cannabis effects
Rimobants blocks the psychoactive and some of the cardiovascular effects of Δ9-Tetrahydrocannabinol in humans without affecting the pharmacokinetics[13].
References
Template:Antiobesity preparations
- ↑ Rimonabant is currently being sold in the United Kingdom by Sanofi-Aventis and in Denmark by Sanofi-Synthelabo under the trade name Acomplia (which is the name used in 18 countries, as of 2007). Riobant, Slimona and Rimoslim are generic forms available in India. If approved in the United States, it is intended to be marketed under the name Zimulti.
- ↑ http://www.acompliareport.com/News/news-061807.htm
- ↑ Article from the Belgian newspaper De Standaard
- ↑ Article from the Swedish TV station TV 4 website
- ↑ "Kassen müssen nicht für "Acomplia" zahlen". tagesschau.de. 2006-10-17. Retrieved 2007-06-13.
- ↑ [2] Neuroprotection by 9-Tetrahydrocannabinol, the Main Active Compound in Marijuana, against Ouabain-Induced In Vivo Excitotoxicity, M. van der Stelt, W. B. Veldhuis, P. R. Bär, G. A. Veldink1, J. F. G. Vliegenthart, and K. Nicolay, The Journal of Neuroscience, September 1, 2001
- ↑ Kim AH, Kerchner GA, and Choi DW. Blocking Excitotoxicity. Chapter 1 in CNS Neuroproteciton. Marcoux FW and Choi DW, editors. Springer, New York. 2002. Pages 3-36
- ↑ BBC NEWS | Health | Suicide risk fears over diet pill
- ↑ http://www.theheart.org/viewArticle.do?primaryKey=913809&nl_id=tho23oct08
- ↑ Cahill K, Ussher M (2007). "Cannabinoid type 1 receptor antagonists (rimonabant) for smoking cessation". Cochrane database of systematic reviews (Online) (4): CD005353. doi:10.1002/14651858.CD005353.pub3. PMID 17943852.
- ↑ Maldonado R, Valverde O, Berrendero F (2006). "Involvement of the endocannabinoid system in drug addiction". Trends Neurosci. 29 (4): 225–32. doi:10.1016/j.tins.2006.01.008. PMID 16483675.
- ↑ Deadwyler SA, Goonawardena AV, Hampson RE (2007). "Short-term memory is modulated by the spontaneous release of endocannabinoids: evidence from hippocampal population codes". Behavioural pharmacology. 18 (5–6): 571–80. doi:10.1097/FBP.0b013e3282ee2adb. PMID 17762525.
- ↑ Huestis MA, Gorelick DA, Heishman SJ; et al. (2001). "Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716". Arch. Gen. Psychiatry. 58 (4): 322–8. PMID 11296091.
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drug has EMA link
- Articles containing unverified chemical infoboxes
- Anorectics
- CB1 receptor antagonists
- Piperidines
- Endocrinology
- Drugs
- CS1 maint: Multiple names: authors list
- CS1 maint: Explicit use of et al.