Rifabutin: Difference between revisions
No edit summary |
No edit summary |
||
| Line 15: | Line 15: | ||
<!--FDA-Labeled Indications and Dosage (Adult)--> | <!--FDA-Labeled Indications and Dosage (Adult)--> | ||
|fdaLIADAdult====== | |fdaLIADAdult======Disseminated infection due to Mycobacterium avium-intracellulare group; Prophylaxis - HIV infection===== | ||
* Dosing Information | * Dosing Information | ||
| Line 21: | Line 21: | ||
:* Dosage | :* Dosage | ||
|offLabelAdultNoGuideSupport======Crohn's disease===== | |||
|offLabelAdultNoGuideSupport====== | |||
* Dosing Information | * Dosing Information | ||
| Line 72: | Line 35: | ||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | <!--FDA-Labeled Indications and Dosage (Pediatric)--> | ||
|contraindications=* Condition1 | |contraindications=* Condition1 | ||
| Line 295: | Line 217: | ||
<!--Drug box 2--> | <!--Drug box 2--> | ||
|drugBox= | |drugBox=[[File:Rifabutin wiki.png|600px|thumbnail|left]] | ||
|mechAction= | {{clear}} | ||
|mechAction=Rifabutin inhibits DNA-dependent RNA polymerase in susceptible strains of Escherichia coli and Bacillus subtilis but not in mammalian cells. In resistant strains of E. coli, rifabutin, like rifampin, did not inhibit this enzyme. It is not known whether rifabutin inhibits DNA-dependent RNA polymerase in Mycobacterium avium or in M. intracellulare which comprise M. avium complex (MAC). | |||
MICROBIOLOGY | |||
Mechanism of Action | |||
Rifabutin inhibits DNA-dependent RNA polymerase in susceptible strains of Escherichia coli and Bacillus subtilis but not in mammalian cells. In resistant strains of E. coli, rifabutin, like rifampin, did not inhibit this enzyme. It is not known whether rifabutin inhibits DNA-dependent RNA polymerase in Mycobacterium avium or in M. intracellulare which comprise M. avium complex (MAC). | |||
Susceptibility Testing | |||
In vitro susceptibility testing methods and diagnostic products used for determining minimum inhibitory concentration (MIC) values against M. avium complex (MAC) organisms have not been standardized. Breakpoints to determine whether clinical isolates of MAC and other mycobacterial species are susceptible or resistant to rifabutin have not been established. | |||
In Vitro Studies | |||
Rifabutin has demonstrated in vitro activity against M. avium complex (MAC) organisms isolated from both HIV-positive and HIV-negative people. While gene probe techniques may be used to identify these two organisms, many reported studies did not distinguish between these two species. The vast majority of isolates from MAC-infected, HIV-positive people are M. avium, whereas in HIV-negative people, about 40% of the MAC isolates are M. intracellulare. | |||
Various in vitro methodologies employing broth or solid media, with and without polysorbate 80 (Tween 80), have been used to determine rifabutin MIC values for mycobacterial species. In general, MIC values determined in broth are several fold lower than that observed with methods employing solid media. Utilization of Tween 80 in these assays has been shown to further lower MIC values. | |||
However, MIC values were substantially higher for egg-based compared to agar-based solid media. | |||
Rifabutin activity against 211 MAC isolates from HIV-positive people was evaluated in vitro utilizing a radiometric broth and an agar dilution method. Results showed that 78% and 82% of these isolates had MIC99 values of ≤0.25 µg/mL and ≤1.0 µg/mL, respectively, when evaluated by these two methods. Rifabutin was also shown to be active against phagocytized, M. avium complex in a mouse macrophage cell culture model. | |||
Rifabutin has in vitro activity against many strains of Mycobacterium tuberculosis. In one study, utilizing the radiometric broth method, each of 17 and 20 rifampin-naive clinical isolates tested from the United States and Taiwan, respectively, were shown to be susceptible to rifabutin concentrations of ≤0.125 µg/mL. | |||
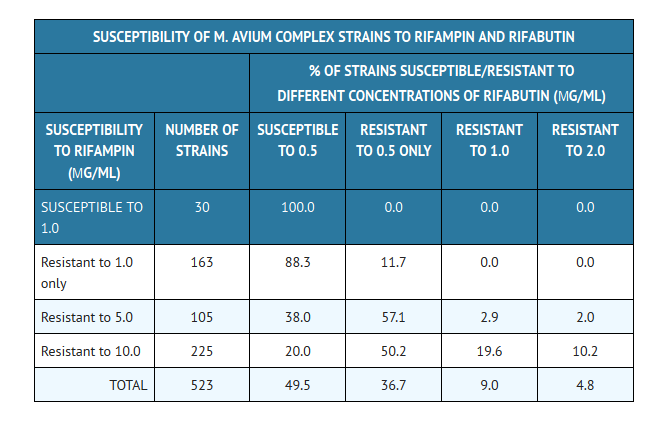
Cross-resistance between rifampin and rifabutin is commonly observed with M. tuberculosis and M. avium complex isolates. Isolates of M. tuberculosis resistant to rifampin are likely to be resistant to rifabutin. Rifampicin and rifabutin MIC99 values against 523 isolates of M. avium complex were determined utilizing the agar dilution method (Ref. Heifets, Leonid B. and Iseman, Michael D. 1985. Determination of in vitro susceptibility of Mycobacteria to Ansamycin. Am. Rev. Respir. Dis. 132 (3):710–711). | |||
[[File:Rifabutin microbiology.png|600px|thumbnail|left]] | |||
{{clear}} | |||
|structure=MYCOBUTIN Capsules contain the antimycobacterial agent rifabutin, which is a semisynthetic ansamycin antibiotic derived from rifamycin S. MYCOBUTIN Capsules for oral administration contain 150 mg of rifabutin, USP, per capsule, along with the inactive ingredients microcrystalline cellulose, magnesium stearate, red iron oxide, silica gel, sodium lauryl sulfate, titanium dioxide, and edible white ink. | |||
The chemical name for rifabutin is 1',4-didehydro-1-deoxy-1,4-dihydro-5'-(2-methylpropyl)-1-oxorifamycin XIV (Chemical Abstracts Service, 9th Collective Index) or (9S, 12E, 14S, 15R, 16S, 17R, 18R, 19R, 20S, 21S, 22E, 24Z)-6,16,18,20-tetrahydroxy-1'-isobutyl-14-methoxy-7,9,15,17,19,21,25-heptamethyl-spiro [9,4-(epoxypentadeca[1,11,13]trienimino)-2H-furo[2',3':7,8]naphth[1,2-d] imidazole-2,4'-piperidine]-5,10,26-(3H,9H)-trione-16-acetate. Rifabutin has a molecular formula of C46H62N4O11, a molecular weight of 847.02 and the following structure: | |||
[[File:Rifabutin structure.png|600px|thumbnail|left]] | |||
{{clear}} | |||
Rifabutin is a red-violet powder soluble in chloroform and methanol, sparingly soluble in ethanol, and very slightly soluble in water (0.19 mg/mL). Its log P value (the base 10 logarithm of the partition coefficient between n-octanol and water) is 3.2 (n-octanol/water). | |||
|PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |PD=There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | ||
<!--Pharmacokinetics--> | <!--Pharmacokinetics--> | ||
|PK= | |PK=Absorption | ||
Following a single oral dose of 300 mg to nine healthy adult volunteers, rifabutin was readily absorbed from the gastrointestinal tract with mean (±SD) peak plasma levels (Cmax) of 375 (±267) ng/mL (range: 141 to 1033 ng/mL) attained in 3.3 (±0.9) hours (Tmax range: 2 to 4 hours). Absolute bioavailability assessed in five HIV-positive patients, who received both oral and intravenous doses, averaged 20%. Total recovery of radioactivity in the urine indicates that at least 53% of the orally administered rifabutin dose is absorbed from the gastrointestinal tract. The bioavailability of rifabutin from the capsule dosage form, relative to an oral solution, was 85% in 12 healthy adult volunteers. High-fat meals slow the rate without influencing the extent of absorption from the capsule dosage form. Plasma concentrations post-Cmax declined in an apparent biphasic manner. Pharmacokinetic dose-proportionality was established over the 300 to 600 mg dose range in nine healthy adult volunteers (crossover design) and in 16 early symptomatic human immunodeficiency virus (HIV)-positive patients over a 300 to 900 mg dose range. | |||
Distribution | |||
Due to its high lipophilicity, rifabutin demonstrates a high propensity for distribution and intracellular tissue uptake. Following intravenous dosing, estimates of apparent steady-state distribution volume (9.3 ± 1.5 L/kg) in five HIV-positive patients exceeded total body water by approximately 15-fold. Substantially higher intracellular tissue levels than those seen in plasma have been observed in both rat and man. The lung-to-plasma concentration ratio, obtained at 12 hours, was approximately 6.5 in four surgical patients who received an oral dose. Mean rifabutin steady-state trough levels (Cp,minss; 24-hour post-dose) ranged from 50 to 65 ng/mL in HIV-positive patients and in healthy adult volunteers. About 85% of the drug is bound in a concentration-independent manner to plasma proteins over a concentration range of 0.05 to 1 µg/mL. Binding does not appear to be influenced by renal or hepatic dysfunction. Rifabutin was slowly eliminated from plasma in seven healthy adult volunteers, presumably because of distribution-limited elimination, with a mean terminal half-life of 45 (±17) hours (range: 16 to 69 hours). Although the systemic levels of rifabutin following multiple dosing decreased by 38%, its terminal half-life remained unchanged. | |||
Metabolism | |||
Of the five metabolites that have been identified, 25-O-desacetyl and 31-hydroxy are the most predominant, and show a plasma metabolite:parent area under the curve ratio of 0.10 and 0.07, respectively. The former has an activity equal to the parent drug and contributes up to 10% to the total antimicrobial activity. | |||
Excretion | |||
A mass-balance study in three healthy adult volunteers with 14C-labeled rifabutin showed that 53% of the oral dose was excreted in the urine, primarily as metabolites. About 30% of the dose is excreted in the feces. Mean systemic clearance (CLs/F) in healthy adult volunteers following a single oral dose was 0.69 (±0.32) L/hr/kg (range: 0.46 to 1.34 L/hr/kg). Renal and biliary clearance of unchanged drug each contribute approximately 5% to CLs/F. | |||
Pharmacokinetics in Special Populations | |||
Geriatric | |||
Compared to healthy volunteers, steady-state kinetics of MYCOBUTIN are more variable in elderly patients (>70 years). | |||
Pediatric | |||
The pharmacokinetics of MYCOBUTIN have not been studied in subjects under 18 years of age. | |||
Renal Insufficiency | |||
The disposition of rifabutin (300 mg) was studied in 18 patients with varying degrees of renal function. Area under plasma concentration time curve (AUC) increased by about 71% in patients with severe renal insufficiency (creatinine clearance below 30 mL/min) compared to patients with creatinine clearance (Crcl) between 61–74 mL/min. In patients with mild to moderate renal insufficiency (Crcl between 30–61 mL/min), the AUC increased by about 41%. A reduction in the dosage of rifabutin is recommended for patients with Crcl< 30 mL/min (see DOSAGE AND ADMINISTRATION). | |||
Drug-Drug Interactions | |||
(see also PRECAUTIONS-Drug Interactions) | |||
Rifabutin induces the enzymes of the cytochrome P450 3A subfamily (CYP3A) and therefore may reduce the plasma concentrations of drugs that are principally metabolized by those enzymes. Rifabutin is also metabolized by CYP3A. Thus, some drugs that inhibit CYP3A may significantly increase plasma concentrations of rifabutin. | |||
Antifungals | |||
Fluconazole | |||
Fluconazole (200 mg/day for 2 weeks) increased the AUC of rifabutin (300 mg/day for 2 weeks) by 82% and Cmax by 88% in 12 HIV-infected patients who were on zidovudine (500 mg/day) maintenance therapy (see PRECAUTIONS-Drug Interactions). Rifabutin did not affect the pharmacokinetics of fluconazole. | |||
Itraconazole | |||
Coadministration of itraconazole (200 mg/day) with rifabutin (300 mg/day) in six HIV-infected patients reduced both the AUC and Cmax of itraconazole by 70% to 75% (see PRECAUTIONS-Drug Interactions). | |||
Antipneumocystis Agents | |||
Dapsone | |||
Rifabutin (300 mg/day) decreased the AUC of dapsone (50 mg/day) in HIV-infected patients (n=16) by about 27% to 40%. | |||
Sulfamethoxazole-trimethoprim | |||
Coadministration of rifabutin (300 mg/day) and sulfamethoxazole-trimethoprim (double strength) in 12 HIV-infected patients decreased the AUC of sulfamethoxazole-trimethoprim by about 15% to 20%. When trimethoprim was given alone, the AUC of trimethoprim was decreased by 14% and the Cmax by 6%. | |||
Sulfamethoxazole-trimethoprim did not alter the pharmacokinetics of rifabutin. | |||
Antiretroviral Agents | |||
Delavirdine | |||
In 7 HIV-infected patients, rifabutin (300 mg/day) decreased delavirdine (400 mg q 8h) AUC by about 80%, Cmax by about 75%, and mean trough plasma concentrations by about 95%. Based on comparisons with historical data, delavirdine appeared to increase the AUC of rifabutin by at least 100% (see PRECAUTIONS-Drug Interactions). | |||
Didanosine | |||
In 12 HIV-infected patients, coadministration of rifabutin (300 or 600 mg/day) and didanosine (167–375 mg BID) did not alter the pharmacokinetics of either drug. | |||
Indinavir | |||
In healthy volunteers, coadministration of indinavir (800 mg q 8h) and rifabutin (300 mg/day) decreased the AUC of indinavir by about 30% and increased the AUC of rifabutin by about 200% (see PRECAUTIONS-Drug Interactions). | |||
Nelfinavir | |||
Coadministration of nelfinavir (750 mg q 8h for 8 days) and rifabutin (300 mg/day for 7–8 days) decreased the AUC and Cmax of nelfinavir by about 32% and 25%, respectively, and increased the AUC and Cmax of rifabutin by about 207% and 146%, respectively (see PRECAUTIONS-Drug Interactions). | |||
Ritonavir | |||
Coadministration of ritonavir (500 mg q 12h) and rifabutin (150 mg/day) increased the AUC and Cmax of rifabutin by more than 400% and 250%, respectively (see PRECAUTIONS-Drug Interactions). | |||
Saquinavir | |||
In 12 HIV-infected patients, rifabutin (300 mg/day) decreased the AUC of saquinavir (600 mg TID) by about 40% (see PRECAUTIONS-Drug Interactions). | |||
Zidovudine | |||
In 16 HIV-infected patients on zidovudine (100 or 200 mg q 4h), rifabutin (300 or 450 mg/day) lowered the Cmax and AUC of zidovudine by about 48% and 32%, respectively. However, zidovudine levels remained within the therapeutic range during coadministration of rifabutin. Zidovudine did not affect the pharmacokinetics of rifabutin. | |||
Antituberculosis Agents | |||
In studies conducted in healthy volunteers, rifabutin (300 mg) did not alter the pharmacokinetics of ethambutol (n=10) or isoniazid (n=10). | |||
Macrolides | |||
Clarithromycin | |||
In studies conducted in HIV-infected patients, coadministration of rifabutin (300 mg/day) and clarithromycin (500 mg q 12h) decreased the AUC of clarithromycin by about 50% (n=12) and increased the AUC of rifabutin by about 75% (n=14) (see PRECAUTIONS-Drug Interactions). | |||
Other Drugs | |||
Methadone | |||
Rifabutin did not alter the pharmacokinetics of methadone in 24 HIV-infected, methadone-maintained, former intravenous drug users. | |||
Oral contraceptives | |||
In 22 healthy female volunteers receiving an oral contraceptive (35 mcg ethinylestradiol (EE) and 1 mg norethindrone (NE) daily for 21 days), rifabutin decreased EE (AUC) and Cmax by 35% and 20%, respectively, and NE AUC by 46% (see PRECAUTIONS-Drug Interactions). | |||
Theophylline | |||
Rifabutin did not alter the pharmacokinetics of theophylline when coadministered in 11 healthy volunteers. | |||
Other drugs | |||
The structurally similar drug, rifampin, is known to reduce the plasma concentrations of a number of other drugs (see prescribing information for rifampin). Although rifabutin is a weaker enzyme inducer than rifampin, rifabutin may be expected to have some effect on those drugs as well. | |||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | ||
<!--Clinical Studies--> | <!--Clinical Studies--> | ||
|clinicalStudies= | |clinicalStudies=Two randomized, double-blind clinical trials (study 023 and study 027) compared MYCOBUTIN (300 mg/day) to placebo in patients with CDC-defined AIDS and CD4 counts ≤ 200 cells/µL. These studies accrued patients from 2/90 through 2/92. Study 023 enrolled 590 patients, with a median CD4 cell count at study entry of 42 cells/µL (mean 61). Study 027 enrolled 556 patients with a median CD4 cell count at study entry of 40 cells/µL (mean 58). | ||
Endpoints included the following: | |||
MAC bacteremia, defined as at least one blood culture positive for Mycobacterium avium complex (MAC) bacteria. | |||
Clinically significant disseminated MAC disease, defined as MAC bacteremia accompanied by signs or symptoms of serious MAC infection, including one or more of the following: fever, night sweats, rigors, weight loss, worsening anemia, and/or elevations in alkaline phosphatase. | |||
Survival | |||
MAC bacteremia | |||
Participants who received MYCOBUTIN were one-third to one-half as likely to develop MAC bacteremia as were participants who received placebo. These results were statistically significant (study 023: p<0.001; study 027: p = 0.002). | |||
In study 023, the one-year cumulative incidence of MAC bacteremia, on an intent to treat basis, was 9% for patients randomized to MYCOBUTIN and 22% for patients randomized to placebo. In study 027, these rates were 13% and 28% for patients receiving MYCOBUTIN and placebo, respectively. | |||
Most cases of MAC bacteremia (approximately 90% in these studies) occurred among participants whose CD4 count at study entry was ≤100 cells/µL. The median and mean CD4 counts at onset of MAC bacteremia were 13 cells/µL and 24 cells/µL, respectively. These studies did not investigate the optimal time to begin MAC prophylaxis. | |||
Clinically significant disseminated MAC disease | |||
In association with the decreased incidence of bacteremia, patients on MYCOBUTIN showed reductions in the signs and symptoms of disseminated MAC disease, including fever, night sweats, weight loss, fatigue, abdominal pain, anemia, and hepatic dysfunction. | |||
Survival | |||
The one-year survival rates in study 023 were 77% for the group receiving MYCOBUTIN and 77% for the placebo group. In study 027, the one-year survival rates were 77% for the group receiving MYCOBUTIN and 70% for the placebo group. | |||
These differences were not statistically significant. | |||
|howSupplied=* | |howSupplied=* | ||
<!--Patient Counseling Information--> | <!--Patient Counseling Information--> | ||
|packLabel=[[File:Rifabutin pdp.png|600px|thumbnail|left]] | |||
{{clear}} | |||
[[File:Rifabutin label.png|600px|thumbnail|left]] | |||
{{clear}} | |||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | ||
Revision as of 16:16, 22 October 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Rifabutin is a anti-bacterial, anti-infective agent that is FDA approved for the prophylaxis of disseminated Mycobacterium avium complex (MAC) disease in patients with advanced HIV infection. Common adverse reactions include discoloration of skin, rash, diarrhea, disorder of taste, indigestion, loss of appetite, nausea, vomiting, increased liver aminotransferase level (mild), ocular discoloration, uveitis, abnormal color of body fluid.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Disseminated infection due to Mycobacterium avium-intracellulare group; Prophylaxis - HIV infection
- Dosing Information
- Dosage
Off-Label Use and Dosage (Adult)
Non–Guideline-Supported Use
Crohn's disease
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Rifabutin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Rifabutin FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Contraindications
- Condition1
Warnings
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Rifabutin in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Rifabutin in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Rifabutin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Rifabutin during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Rifabutin with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Rifabutin with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Rifabutin with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Rifabutin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Rifabutin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Rifabutin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Rifabutin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Rifabutin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Rifabutin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Rifabutin in the drug label.
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Rifabutin in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Rifabutin in the drug label.
Pharmacology
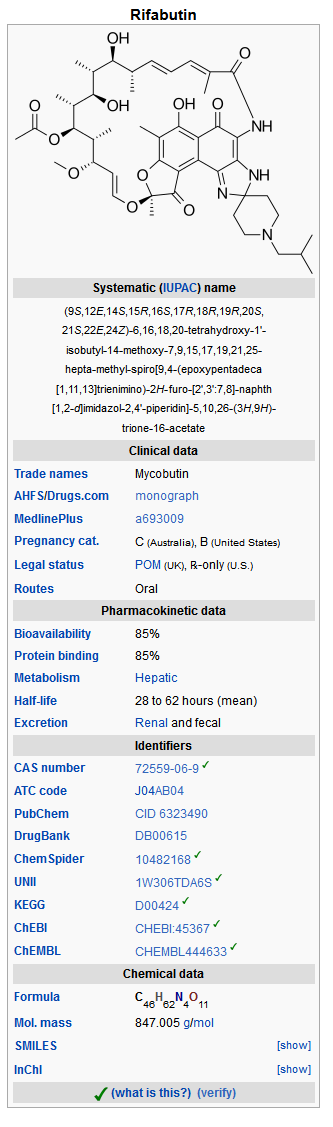
Mechanism of Action
Rifabutin inhibits DNA-dependent RNA polymerase in susceptible strains of Escherichia coli and Bacillus subtilis but not in mammalian cells. In resistant strains of E. coli, rifabutin, like rifampin, did not inhibit this enzyme. It is not known whether rifabutin inhibits DNA-dependent RNA polymerase in Mycobacterium avium or in M. intracellulare which comprise M. avium complex (MAC).
MICROBIOLOGY
Mechanism of Action
Rifabutin inhibits DNA-dependent RNA polymerase in susceptible strains of Escherichia coli and Bacillus subtilis but not in mammalian cells. In resistant strains of E. coli, rifabutin, like rifampin, did not inhibit this enzyme. It is not known whether rifabutin inhibits DNA-dependent RNA polymerase in Mycobacterium avium or in M. intracellulare which comprise M. avium complex (MAC).
Susceptibility Testing
In vitro susceptibility testing methods and diagnostic products used for determining minimum inhibitory concentration (MIC) values against M. avium complex (MAC) organisms have not been standardized. Breakpoints to determine whether clinical isolates of MAC and other mycobacterial species are susceptible or resistant to rifabutin have not been established.
In Vitro Studies
Rifabutin has demonstrated in vitro activity against M. avium complex (MAC) organisms isolated from both HIV-positive and HIV-negative people. While gene probe techniques may be used to identify these two organisms, many reported studies did not distinguish between these two species. The vast majority of isolates from MAC-infected, HIV-positive people are M. avium, whereas in HIV-negative people, about 40% of the MAC isolates are M. intracellulare.
Various in vitro methodologies employing broth or solid media, with and without polysorbate 80 (Tween 80), have been used to determine rifabutin MIC values for mycobacterial species. In general, MIC values determined in broth are several fold lower than that observed with methods employing solid media. Utilization of Tween 80 in these assays has been shown to further lower MIC values.
However, MIC values were substantially higher for egg-based compared to agar-based solid media.
Rifabutin activity against 211 MAC isolates from HIV-positive people was evaluated in vitro utilizing a radiometric broth and an agar dilution method. Results showed that 78% and 82% of these isolates had MIC99 values of ≤0.25 µg/mL and ≤1.0 µg/mL, respectively, when evaluated by these two methods. Rifabutin was also shown to be active against phagocytized, M. avium complex in a mouse macrophage cell culture model.
Rifabutin has in vitro activity against many strains of Mycobacterium tuberculosis. In one study, utilizing the radiometric broth method, each of 17 and 20 rifampin-naive clinical isolates tested from the United States and Taiwan, respectively, were shown to be susceptible to rifabutin concentrations of ≤0.125 µg/mL.
Cross-resistance between rifampin and rifabutin is commonly observed with M. tuberculosis and M. avium complex isolates. Isolates of M. tuberculosis resistant to rifampin are likely to be resistant to rifabutin. Rifampicin and rifabutin MIC99 values against 523 isolates of M. avium complex were determined utilizing the agar dilution method (Ref. Heifets, Leonid B. and Iseman, Michael D. 1985. Determination of in vitro susceptibility of Mycobacteria to Ansamycin. Am. Rev. Respir. Dis. 132 (3):710–711).
Structure
MYCOBUTIN Capsules contain the antimycobacterial agent rifabutin, which is a semisynthetic ansamycin antibiotic derived from rifamycin S. MYCOBUTIN Capsules for oral administration contain 150 mg of rifabutin, USP, per capsule, along with the inactive ingredients microcrystalline cellulose, magnesium stearate, red iron oxide, silica gel, sodium lauryl sulfate, titanium dioxide, and edible white ink.
The chemical name for rifabutin is 1',4-didehydro-1-deoxy-1,4-dihydro-5'-(2-methylpropyl)-1-oxorifamycin XIV (Chemical Abstracts Service, 9th Collective Index) or (9S, 12E, 14S, 15R, 16S, 17R, 18R, 19R, 20S, 21S, 22E, 24Z)-6,16,18,20-tetrahydroxy-1'-isobutyl-14-methoxy-7,9,15,17,19,21,25-heptamethyl-spiro [9,4-(epoxypentadeca[1,11,13]trienimino)-2H-furo[2',3':7,8]naphth[1,2-d] imidazole-2,4'-piperidine]-5,10,26-(3H,9H)-trione-16-acetate. Rifabutin has a molecular formula of C46H62N4O11, a molecular weight of 847.02 and the following structure:
Rifabutin is a red-violet powder soluble in chloroform and methanol, sparingly soluble in ethanol, and very slightly soluble in water (0.19 mg/mL). Its log P value (the base 10 logarithm of the partition coefficient between n-octanol and water) is 3.2 (n-octanol/water).
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Rifabutin in the drug label.
Pharmacokinetics
Absorption
Following a single oral dose of 300 mg to nine healthy adult volunteers, rifabutin was readily absorbed from the gastrointestinal tract with mean (±SD) peak plasma levels (Cmax) of 375 (±267) ng/mL (range: 141 to 1033 ng/mL) attained in 3.3 (±0.9) hours (Tmax range: 2 to 4 hours). Absolute bioavailability assessed in five HIV-positive patients, who received both oral and intravenous doses, averaged 20%. Total recovery of radioactivity in the urine indicates that at least 53% of the orally administered rifabutin dose is absorbed from the gastrointestinal tract. The bioavailability of rifabutin from the capsule dosage form, relative to an oral solution, was 85% in 12 healthy adult volunteers. High-fat meals slow the rate without influencing the extent of absorption from the capsule dosage form. Plasma concentrations post-Cmax declined in an apparent biphasic manner. Pharmacokinetic dose-proportionality was established over the 300 to 600 mg dose range in nine healthy adult volunteers (crossover design) and in 16 early symptomatic human immunodeficiency virus (HIV)-positive patients over a 300 to 900 mg dose range.
Distribution
Due to its high lipophilicity, rifabutin demonstrates a high propensity for distribution and intracellular tissue uptake. Following intravenous dosing, estimates of apparent steady-state distribution volume (9.3 ± 1.5 L/kg) in five HIV-positive patients exceeded total body water by approximately 15-fold. Substantially higher intracellular tissue levels than those seen in plasma have been observed in both rat and man. The lung-to-plasma concentration ratio, obtained at 12 hours, was approximately 6.5 in four surgical patients who received an oral dose. Mean rifabutin steady-state trough levels (Cp,minss; 24-hour post-dose) ranged from 50 to 65 ng/mL in HIV-positive patients and in healthy adult volunteers. About 85% of the drug is bound in a concentration-independent manner to plasma proteins over a concentration range of 0.05 to 1 µg/mL. Binding does not appear to be influenced by renal or hepatic dysfunction. Rifabutin was slowly eliminated from plasma in seven healthy adult volunteers, presumably because of distribution-limited elimination, with a mean terminal half-life of 45 (±17) hours (range: 16 to 69 hours). Although the systemic levels of rifabutin following multiple dosing decreased by 38%, its terminal half-life remained unchanged.
Metabolism
Of the five metabolites that have been identified, 25-O-desacetyl and 31-hydroxy are the most predominant, and show a plasma metabolite:parent area under the curve ratio of 0.10 and 0.07, respectively. The former has an activity equal to the parent drug and contributes up to 10% to the total antimicrobial activity.
Excretion
A mass-balance study in three healthy adult volunteers with 14C-labeled rifabutin showed that 53% of the oral dose was excreted in the urine, primarily as metabolites. About 30% of the dose is excreted in the feces. Mean systemic clearance (CLs/F) in healthy adult volunteers following a single oral dose was 0.69 (±0.32) L/hr/kg (range: 0.46 to 1.34 L/hr/kg). Renal and biliary clearance of unchanged drug each contribute approximately 5% to CLs/F.
Pharmacokinetics in Special Populations
Geriatric
Compared to healthy volunteers, steady-state kinetics of MYCOBUTIN are more variable in elderly patients (>70 years).
Pediatric
The pharmacokinetics of MYCOBUTIN have not been studied in subjects under 18 years of age.
Renal Insufficiency
The disposition of rifabutin (300 mg) was studied in 18 patients with varying degrees of renal function. Area under plasma concentration time curve (AUC) increased by about 71% in patients with severe renal insufficiency (creatinine clearance below 30 mL/min) compared to patients with creatinine clearance (Crcl) between 61–74 mL/min. In patients with mild to moderate renal insufficiency (Crcl between 30–61 mL/min), the AUC increased by about 41%. A reduction in the dosage of rifabutin is recommended for patients with Crcl< 30 mL/min (see DOSAGE AND ADMINISTRATION).
Drug-Drug Interactions
(see also PRECAUTIONS-Drug Interactions)
Rifabutin induces the enzymes of the cytochrome P450 3A subfamily (CYP3A) and therefore may reduce the plasma concentrations of drugs that are principally metabolized by those enzymes. Rifabutin is also metabolized by CYP3A. Thus, some drugs that inhibit CYP3A may significantly increase plasma concentrations of rifabutin.
Antifungals
Fluconazole
Fluconazole (200 mg/day for 2 weeks) increased the AUC of rifabutin (300 mg/day for 2 weeks) by 82% and Cmax by 88% in 12 HIV-infected patients who were on zidovudine (500 mg/day) maintenance therapy (see PRECAUTIONS-Drug Interactions). Rifabutin did not affect the pharmacokinetics of fluconazole.
Itraconazole
Coadministration of itraconazole (200 mg/day) with rifabutin (300 mg/day) in six HIV-infected patients reduced both the AUC and Cmax of itraconazole by 70% to 75% (see PRECAUTIONS-Drug Interactions).
Antipneumocystis Agents
Dapsone
Rifabutin (300 mg/day) decreased the AUC of dapsone (50 mg/day) in HIV-infected patients (n=16) by about 27% to 40%.
Sulfamethoxazole-trimethoprim
Coadministration of rifabutin (300 mg/day) and sulfamethoxazole-trimethoprim (double strength) in 12 HIV-infected patients decreased the AUC of sulfamethoxazole-trimethoprim by about 15% to 20%. When trimethoprim was given alone, the AUC of trimethoprim was decreased by 14% and the Cmax by 6%.
Sulfamethoxazole-trimethoprim did not alter the pharmacokinetics of rifabutin.
Antiretroviral Agents
Delavirdine
In 7 HIV-infected patients, rifabutin (300 mg/day) decreased delavirdine (400 mg q 8h) AUC by about 80%, Cmax by about 75%, and mean trough plasma concentrations by about 95%. Based on comparisons with historical data, delavirdine appeared to increase the AUC of rifabutin by at least 100% (see PRECAUTIONS-Drug Interactions).
Didanosine
In 12 HIV-infected patients, coadministration of rifabutin (300 or 600 mg/day) and didanosine (167–375 mg BID) did not alter the pharmacokinetics of either drug.
Indinavir
In healthy volunteers, coadministration of indinavir (800 mg q 8h) and rifabutin (300 mg/day) decreased the AUC of indinavir by about 30% and increased the AUC of rifabutin by about 200% (see PRECAUTIONS-Drug Interactions).
Nelfinavir
Coadministration of nelfinavir (750 mg q 8h for 8 days) and rifabutin (300 mg/day for 7–8 days) decreased the AUC and Cmax of nelfinavir by about 32% and 25%, respectively, and increased the AUC and Cmax of rifabutin by about 207% and 146%, respectively (see PRECAUTIONS-Drug Interactions).
Ritonavir
Coadministration of ritonavir (500 mg q 12h) and rifabutin (150 mg/day) increased the AUC and Cmax of rifabutin by more than 400% and 250%, respectively (see PRECAUTIONS-Drug Interactions).
Saquinavir
In 12 HIV-infected patients, rifabutin (300 mg/day) decreased the AUC of saquinavir (600 mg TID) by about 40% (see PRECAUTIONS-Drug Interactions).
Zidovudine
In 16 HIV-infected patients on zidovudine (100 or 200 mg q 4h), rifabutin (300 or 450 mg/day) lowered the Cmax and AUC of zidovudine by about 48% and 32%, respectively. However, zidovudine levels remained within the therapeutic range during coadministration of rifabutin. Zidovudine did not affect the pharmacokinetics of rifabutin.
Antituberculosis Agents
In studies conducted in healthy volunteers, rifabutin (300 mg) did not alter the pharmacokinetics of ethambutol (n=10) or isoniazid (n=10).
Macrolides
Clarithromycin
In studies conducted in HIV-infected patients, coadministration of rifabutin (300 mg/day) and clarithromycin (500 mg q 12h) decreased the AUC of clarithromycin by about 50% (n=12) and increased the AUC of rifabutin by about 75% (n=14) (see PRECAUTIONS-Drug Interactions).
Other Drugs
Methadone
Rifabutin did not alter the pharmacokinetics of methadone in 24 HIV-infected, methadone-maintained, former intravenous drug users.
Oral contraceptives
In 22 healthy female volunteers receiving an oral contraceptive (35 mcg ethinylestradiol (EE) and 1 mg norethindrone (NE) daily for 21 days), rifabutin decreased EE (AUC) and Cmax by 35% and 20%, respectively, and NE AUC by 46% (see PRECAUTIONS-Drug Interactions).
Theophylline
Rifabutin did not alter the pharmacokinetics of theophylline when coadministered in 11 healthy volunteers.
Other drugs
The structurally similar drug, rifampin, is known to reduce the plasma concentrations of a number of other drugs (see prescribing information for rifampin). Although rifabutin is a weaker enzyme inducer than rifampin, rifabutin may be expected to have some effect on those drugs as well.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Rifabutin in the drug label.
Clinical Studies
Two randomized, double-blind clinical trials (study 023 and study 027) compared MYCOBUTIN (300 mg/day) to placebo in patients with CDC-defined AIDS and CD4 counts ≤ 200 cells/µL. These studies accrued patients from 2/90 through 2/92. Study 023 enrolled 590 patients, with a median CD4 cell count at study entry of 42 cells/µL (mean 61). Study 027 enrolled 556 patients with a median CD4 cell count at study entry of 40 cells/µL (mean 58).
Endpoints included the following:
MAC bacteremia, defined as at least one blood culture positive for Mycobacterium avium complex (MAC) bacteria. Clinically significant disseminated MAC disease, defined as MAC bacteremia accompanied by signs or symptoms of serious MAC infection, including one or more of the following: fever, night sweats, rigors, weight loss, worsening anemia, and/or elevations in alkaline phosphatase. Survival
MAC bacteremia
Participants who received MYCOBUTIN were one-third to one-half as likely to develop MAC bacteremia as were participants who received placebo. These results were statistically significant (study 023: p<0.001; study 027: p = 0.002).
In study 023, the one-year cumulative incidence of MAC bacteremia, on an intent to treat basis, was 9% for patients randomized to MYCOBUTIN and 22% for patients randomized to placebo. In study 027, these rates were 13% and 28% for patients receiving MYCOBUTIN and placebo, respectively.
Most cases of MAC bacteremia (approximately 90% in these studies) occurred among participants whose CD4 count at study entry was ≤100 cells/µL. The median and mean CD4 counts at onset of MAC bacteremia were 13 cells/µL and 24 cells/µL, respectively. These studies did not investigate the optimal time to begin MAC prophylaxis.
Clinically significant disseminated MAC disease
In association with the decreased incidence of bacteremia, patients on MYCOBUTIN showed reductions in the signs and symptoms of disseminated MAC disease, including fever, night sweats, weight loss, fatigue, abdominal pain, anemia, and hepatic dysfunction.
Survival
The one-year survival rates in study 023 were 77% for the group receiving MYCOBUTIN and 77% for the placebo group. In study 027, the one-year survival rates were 77% for the group receiving MYCOBUTIN and 70% for the placebo group.
These differences were not statistically significant.
How Supplied
Storage
There is limited information regarding Rifabutin Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Rifabutin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Rifabutin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Rifabutin in the drug label.
Precautions with Alcohol
- Alcohol-Rifabutin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Rifabutin
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Rifabutin |Label Name=Rifabutin11.png
}}
{{#subobject:
|Label Page=Rifabutin |Label Name=Rifabutin11.png
}}