Reperfusion injury pathophysiology: Difference between revisions
No edit summary |
|||
| Line 2: | Line 2: | ||
{{Reperfusion injury}} | {{Reperfusion injury}} | ||
'''Editors-In-Chief:''' {{AC}}; [[C. Michael Gibson]], M.S., M.D. [mailto:Mgibson@perfuse.org]; [[User:Kashish Goel|Kashish Goel,M.D.]] | '''Editors-In-Chief:''' {{AC}}; [[C. Michael Gibson]], M.S., M.D. [mailto:Mgibson@perfuse.org]; Dr. Shivam Singla M.D [1], [[User:Kashish Goel|Kashish Goel,M.D.,]] | ||
==Overview== | ==Overview== | ||
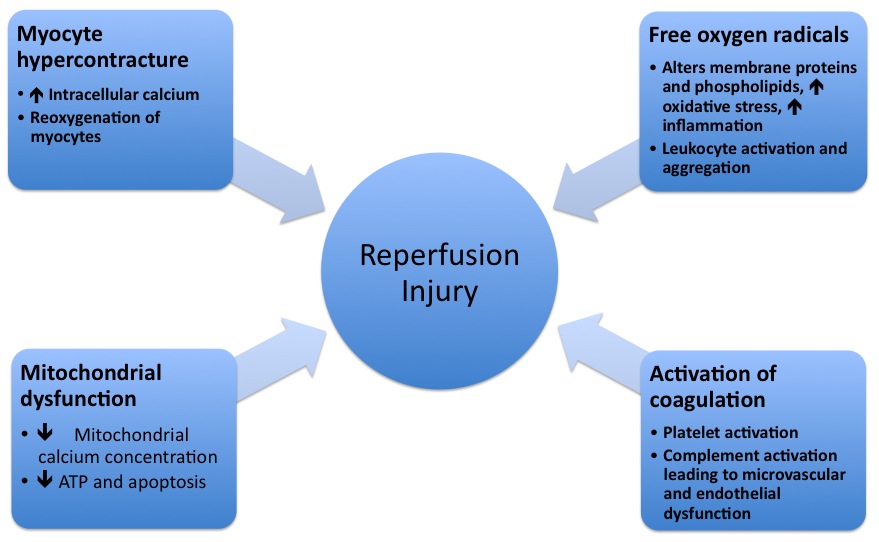
The pathophysiologic mechanisms underlying reperfusion injury include infarction, inflammation, generation of free radicals, an increase in intracellular calcium, development of edema, mitochodrial damage and activation of coagulation. | The pathophysiologic mechanisms underlying reperfusion injury include infarction, inflammation, generation of free radicals, an increase in intracellular calcium, development of edema, mitochodrial damage and activation of coagulation. | ||
| Line 11: | Line 11: | ||
'''Reperfusion injury''' occurs after reinstating the flow to myocardium after a period of reduced oxygen delivery. | '''Reperfusion injury''' occurs after reinstating the flow to myocardium after a period of reduced oxygen delivery. | ||
The damage of reperfusion injury is due in part to the [[inflammatory response]] of damaged tissues. [[White blood cell]]s carried to the area by the newly returning blood release a host of [[cytokine|inflammatory factors]] such as [[interleukin]]s as well as [[reactive oxygen species|free radicals]] in response to tissue damage | The damage of reperfusion injury is due in part to the [[inflammatory response]] of damaged tissues. [[White blood cell]]s carried to the area by the newly returning blood release a host of [[cytokine|inflammatory factors]] such as [[interleukin]]s as well as [[reactive oxygen species|free radicals]] in response to tissue damage | ||
. The restored blood flow reintroduces oxygen within [[cell (biology)|cell]]s that damages cellular [[protein]]s, [[DNA]], and the [[plasma membrane]]. Damage to the cell's membrane may in turn cause the release of more free radicals. Such reactive species may also act indirectly in [[redox signaling]] to turn on [[apoptosis]]. Leukocytes may also build up in small [[capillary|capillaries]], obstructing them and leading to more ischemia<ref name="WMClark" />. | |||
Mitochondrial dysfunction plays an important role in reperfusion injury. While the mitochondrial membrane is usually impermeable to ions and metabolites, ischemia alters permeability by elevating intro-mitochondrial calcium concentrations, reducing [[adenine]] nucleotide concentrations, and causing oxidative stress. This primes the mitochondrial permeability transition pore ([[Mitochondrial permeability transition|MPTP]]), which opens when reperfusion occurs | Mitochondrial dysfunction plays an important role in reperfusion injury. While the mitochondrial membrane is usually impermeable to ions and metabolites, ischemia alters permeability by elevating intro-mitochondrial calcium concentrations, reducing [[adenine]] nucleotide concentrations, and causing oxidative stress. This primes the mitochondrial permeability transition pore ([[Mitochondrial permeability transition|MPTP]]), which opens when reperfusion occurs. This leads to an increased osmotic load into the mitochondrial body causing swelling and rupture, release of mitochondrial proteins which stimulate apoptosis. Mithochondrial function is disrupted and [[ATP]] is hydrolyzed, leading to the activation of degradative enzymes. Finally, excessive [[Poly ADP ribose polymerase]]-1 (PARP-1) activation impairs the function of other organelles and accelerates the production of reactive oxygen species. | ||
In prolonged ischemia (60 minutes or more), [[hypoxanthine]] is formed as breakdown product of [[Adenosine triphosphate|ATP]] metabolism. The enzyme ''[[xanthine dehydrogenase]]'' is converted to ''[[xanthine oxidase]]'' as a result of the higher availability of oxygen. This oxidation results in molecular oxygen being converted into highly reactive [[superoxide]] and [[hydroxyl]] [[Radical (chemistry)|radicals]]. Xanthine oxidase also produces [[uric acid]], which may act as both a prooxidant and as a scavenger of reactive species such as peroxinitrite. Excessive [[nitric oxide]] produced during reperfusion reacts with [[superoxide]] to produce the potent reactive species [[peroxynitrite]]. Such radicals and reactive oxygen species attack cell membrane lipids, proteins, and glycosaminoglycans, causing further damage. They may also initiate specific biological processes by [[redox signaling]]. | In prolonged ischemia (60 minutes or more), [[hypoxanthine]] is formed as breakdown product of [[Adenosine triphosphate|ATP]] metabolism. The enzyme ''[[xanthine dehydrogenase]]'' is converted to ''[[xanthine oxidase]]'' as a result of the higher availability of oxygen. This oxidation results in molecular oxygen being converted into highly reactive [[superoxide]] and [[hydroxyl]] [[Radical (chemistry)|radicals]]. Xanthine oxidase also produces [[uric acid]], which may act as both a prooxidant and as a scavenger of reactive species such as peroxinitrite. Excessive [[nitric oxide]] produced during reperfusion reacts with [[superoxide]] to produce the potent reactive species [[peroxynitrite]]. Such radicals and reactive oxygen species attack cell membrane lipids, proteins, and glycosaminoglycans, causing further damage. They may also initiate specific biological processes by [[redox signaling]]. | ||
| Line 19: | Line 19: | ||
==Specific organs affected by reperfusion injury== | ==Specific organs affected by reperfusion injury== | ||
===The central nervous system=== | ===The central nervous system=== | ||
Reperfusion injury plays a part in the [[brain]]'s [[ischemic cascade]], which is involved in [[stroke]] and [[brain trauma]]. Repeated bouts of ischemia and reperfusion injury also are thought to be a factor leading to the formation and failure to [[wound healing|heal]] of [[chronic wound]]s such as [[pressure sore]]s and [[diabetic foot]] [[ulcer]]s | Reperfusion injury plays a part in the [[brain]]'s [[ischemic cascade]], which is involved in [[stroke]] and [[brain trauma]]. Repeated bouts of ischemia and reperfusion injury also are thought to be a factor leading to the formation and failure to [[wound healing|heal]] of [[chronic wound]]s such as [[pressure sore]]s and [[diabetic foot]] [[ulcer]]s. Continuous pressure limits blood supply and causes ischemia, and the inflammation occurs during reperfusion. As this process is repeated, it eventually damages tissue enough to cause a [[wound]]<ref name="TMustoe" />. | ||
===The myocardium=== | ===The myocardium=== | ||
Restoration of epicardial patency can be associated with reperfusion injury in the myocardium. This can manifest in a number of ways clinically, including arrhythmia, microvascular dysfunction, myocardial stunning, and myocyte death. | Restoration of epicardial patency can be associated with reperfusion injury in the myocardium. This can manifest in a number of ways clinically, including arrhythmia, microvascular dysfunction, myocardial stunning, and myocyte death. | ||
Arrhythmia is mediated by mitochondrial dysfunction, as discussed above. The mitochondrion is unable to restore its inner membrane potential, leading to destabalization of the action potential | Arrhythmia is mediated by mitochondrial dysfunction, as discussed above. The mitochondrion is unable to restore its inner membrane potential, leading to destabalization of the action potential. | ||
Microvascular dysfunction, or "no reflow," as well as myocardial stunning, are both possible consequences of reperfusion injury. Myocardial stunning, which results from persistent anearobic metabolism that continues after reperfusion, may to some extent be mediated by impaired microvascular function | Microvascular dysfunction, or "no reflow," as well as myocardial stunning, are both possible consequences of reperfusion injury. Myocardial stunning, which results from persistent anearobic metabolism that continues after reperfusion, may to some extent be mediated by impaired microvascular function. | ||
An area of ongoing study is how much damage, or myocyte death, is attributable to ischemia vs. reperfusion injury after vessel patency has been established. Animal studies suggest that up to 50% of of infarct size can be related to reperfusion injury | An area of ongoing study is how much damage, or myocyte death, is attributable to ischemia vs. reperfusion injury after vessel patency has been established. Animal studies suggest that up to 50% of of infarct size can be related to reperfusion injury. This opens the door for novel therapies that can attenuate myocyte death due to reperfusion injury. | ||
==References== | ==References== | ||
| Line 38: | Line 38: | ||
[[Category:Up-To-Date]] | [[Category:Up-To-Date]] | ||
[[Category:Up-To-Date cardiology]] | [[Category:Up-To-Date cardiology]] | ||
<references /> | |||
Revision as of 23:26, 11 August 2020
|
Reperfusion injury Microchapters |
|
Treatment |
|---|
|
Reperfusion injury pathophysiology On the Web |
|
American Roentgen Ray Society Images of Reperfusion injury pathophysiology |
|
Risk calculators and risk factors for Reperfusion injury pathophysiology |
Editors-In-Chief: Anjan K. Chakrabarti, M.D. [1]; C. Michael Gibson, M.S., M.D. [2]; Dr. Shivam Singla M.D [1], Kashish Goel,M.D.,
Overview
The pathophysiologic mechanisms underlying reperfusion injury include infarction, inflammation, generation of free radicals, an increase in intracellular calcium, development of edema, mitochodrial damage and activation of coagulation.
Mechanisms of reperfusion injury
Reperfusion injury occurs after reinstating the flow to myocardium after a period of reduced oxygen delivery. The damage of reperfusion injury is due in part to the inflammatory response of damaged tissues. White blood cells carried to the area by the newly returning blood release a host of inflammatory factors such as interleukins as well as free radicals in response to tissue damage . The restored blood flow reintroduces oxygen within cells that damages cellular proteins, DNA, and the plasma membrane. Damage to the cell's membrane may in turn cause the release of more free radicals. Such reactive species may also act indirectly in redox signaling to turn on apoptosis. Leukocytes may also build up in small capillaries, obstructing them and leading to more ischemia[1].
Mitochondrial dysfunction plays an important role in reperfusion injury. While the mitochondrial membrane is usually impermeable to ions and metabolites, ischemia alters permeability by elevating intro-mitochondrial calcium concentrations, reducing adenine nucleotide concentrations, and causing oxidative stress. This primes the mitochondrial permeability transition pore (MPTP), which opens when reperfusion occurs. This leads to an increased osmotic load into the mitochondrial body causing swelling and rupture, release of mitochondrial proteins which stimulate apoptosis. Mithochondrial function is disrupted and ATP is hydrolyzed, leading to the activation of degradative enzymes. Finally, excessive Poly ADP ribose polymerase-1 (PARP-1) activation impairs the function of other organelles and accelerates the production of reactive oxygen species.
In prolonged ischemia (60 minutes or more), hypoxanthine is formed as breakdown product of ATP metabolism. The enzyme xanthine dehydrogenase is converted to xanthine oxidase as a result of the higher availability of oxygen. This oxidation results in molecular oxygen being converted into highly reactive superoxide and hydroxyl radicals. Xanthine oxidase also produces uric acid, which may act as both a prooxidant and as a scavenger of reactive species such as peroxinitrite. Excessive nitric oxide produced during reperfusion reacts with superoxide to produce the potent reactive species peroxynitrite. Such radicals and reactive oxygen species attack cell membrane lipids, proteins, and glycosaminoglycans, causing further damage. They may also initiate specific biological processes by redox signaling.
Specific organs affected by reperfusion injury
The central nervous system
Reperfusion injury plays a part in the brain's ischemic cascade, which is involved in stroke and brain trauma. Repeated bouts of ischemia and reperfusion injury also are thought to be a factor leading to the formation and failure to heal of chronic wounds such as pressure sores and diabetic foot ulcers. Continuous pressure limits blood supply and causes ischemia, and the inflammation occurs during reperfusion. As this process is repeated, it eventually damages tissue enough to cause a wound[2].
The myocardium
Restoration of epicardial patency can be associated with reperfusion injury in the myocardium. This can manifest in a number of ways clinically, including arrhythmia, microvascular dysfunction, myocardial stunning, and myocyte death.
Arrhythmia is mediated by mitochondrial dysfunction, as discussed above. The mitochondrion is unable to restore its inner membrane potential, leading to destabalization of the action potential.
Microvascular dysfunction, or "no reflow," as well as myocardial stunning, are both possible consequences of reperfusion injury. Myocardial stunning, which results from persistent anearobic metabolism that continues after reperfusion, may to some extent be mediated by impaired microvascular function.
An area of ongoing study is how much damage, or myocyte death, is attributable to ischemia vs. reperfusion injury after vessel patency has been established. Animal studies suggest that up to 50% of of infarct size can be related to reperfusion injury. This opens the door for novel therapies that can attenuate myocyte death due to reperfusion injury.