Remifentanil: Difference between revisions
No edit summary |
No edit summary |
||
| Line 55: | Line 55: | ||
ULTIVA should not be administered into the same IV tubing with blood due to potential inactivation by nonspecific esterases in blood products. | ULTIVA should not be administered into the same IV tubing with blood due to potential inactivation by nonspecific esterases in blood products. | ||
|clinicalTrials=ULTIVA produces adverse events that are characteristic of µ-opioids, such as respiratory depression, bradycardia, hypotension, and skeletal muscle rigidity. These adverse events dissipate within minutes of discontinuing or decreasing the infusion rate of ULTIVA. See CLINICAL PHARMACOLOGY, WARNINGS, and PRECAUTIONS on the management of these events. | |clinicalTrials=ULTIVA produces adverse events that are characteristic of µ-opioids, such as respiratory depression, bradycardia, hypotension, and skeletal muscle rigidity. These adverse events dissipate within minutes of discontinuing or decreasing the infusion rate of ULTIVA. See CLINICAL PHARMACOLOGY, WARNINGS, and PRECAUTIONS on the management of these events. | ||
| Line 65: | Line 63: | ||
Approximately 2770 adult patients were exposed to ULTIVA in controlled clinical trials. The frequencies of adverse events during general anesthesia with the recommended doses of ULTIVA are given in Table 3. Each patient was counted once for each type of adverse event. | Approximately 2770 adult patients were exposed to ULTIVA in controlled clinical trials. The frequencies of adverse events during general anesthesia with the recommended doses of ULTIVA are given in Table 3. Each patient was counted once for each type of adverse event. | ||
[[File: | [[File:Remifentanil adverse 1.png|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | ||
The frequencies of adverse events from the clinical studies at the recommended doses of ULTIVA in monitored anesthesia care are given in Table 5. | The frequencies of adverse events from the clinical studies at the recommended doses of ULTIVA in monitored anesthesia care are given in Table 5. | ||
Other Adverse Events in Adult Patients | Other Adverse Events in Adult Patients | ||
| Line 103: | Line 99: | ||
The frequencies of adverse events from the clinical studies at the recommended doses of ULTIVA in cardiac surgery are given in Tables 6, 7, and 8. These tables represent adverse events collected during discrete phases of cardiac surgery. Any event should be viewed as temporally associated with drug administration and the phase indicated should not be perceived as the only time the event might occur. | The frequencies of adverse events from the clinical studies at the recommended doses of ULTIVA in cardiac surgery are given in Tables 6, 7, and 8. These tables represent adverse events collected during discrete phases of cardiac surgery. Any event should be viewed as temporally associated with drug administration and the phase indicated should not be perceived as the only time the event might occur. | ||
[[File: | [[File:Remifentanil Adverse 2.png|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | ||
Pediatrics | Pediatrics | ||
| Line 111: | Line 107: | ||
The frequencies of adverse events during general anesthesia with the recommended doses of ULTIVA are given in Table 9. Each patient was counted once for each type of adverse event. There were no adverse events ≥1% for any treatment group during the maintenance period in the pediatric patient general anesthesia studies. | The frequencies of adverse events during general anesthesia with the recommended doses of ULTIVA are given in Table 9. Each patient was counted once for each type of adverse event. There were no adverse events ≥1% for any treatment group during the maintenance period in the pediatric patient general anesthesia studies. | ||
[[File: | [[File:REMIFENTANILAdverse 3.jpg|thumb|none|400px|left|This image is provided by the National Library of Medicine.]] | ||
Observed During Clinical Practice | Observed During Clinical Practice | ||
| Line 122: | Line 118: | ||
|mechAction=ULTIVA is a µ-opioid agonist with rapid onset and peak effect, and short duration of action. The µ-opioid activity of ULTIVA is antagonized by opioid antagonists such as naloxone. | |mechAction=ULTIVA is a µ-opioid agonist with rapid onset and peak effect, and short duration of action. The µ-opioid activity of ULTIVA is antagonized by opioid antagonists such as naloxone. | ||
| Line 190: | Line 184: | ||
The pharmacodynamics of ULTIVA (ventilatory response to hypercarbia) are unaltered in patients with severe hepatic dysfunction awaiting liver transplant. | The pharmacodynamics of ULTIVA (ventilatory response to hypercarbia) are unaltered in patients with severe hepatic dysfunction awaiting liver transplant. | ||
|PK=After IV doses administered over 60 seconds, the pharmacokinetics of remifentanil fit a three-compartment model with a rapid distribution half-life of 1 minute, a slower distribution half-life of 6 minutes, and a terminal elimination half-life of 10 to 20 minutes. Since the terminal elimination component contributes less than 10% of the overall area under the concentration versus time curve (AUC), the effective biological half-life of ULTIVA is 3 to 10 minutes. This is similar to the 3- to 10-minute half-life measured after termination of prolonged infusions (up to 4 hours; see Figure 2) and correlates with recovery times observed in the clinical setting after infusions up to 12 hours. Concentrations of remifentanil are proportional to the dose administered throughout the recommended dose range. The pharmacokinetics of remifentanil are unaffected by the presence of renal or hepatic impairment. | |PK=After IV doses administered over 60 seconds, the pharmacokinetics of remifentanil fit a three-compartment model with a rapid distribution half-life of 1 minute, a slower distribution half-life of 6 minutes, and a terminal elimination half-life of 10 to 20 minutes. Since the terminal elimination component contributes less than 10% of the overall area under the concentration versus time curve (AUC), the effective biological half-life of ULTIVA is 3 to 10 minutes. This is similar to the 3- to 10-minute half-life measured after termination of prolonged infusions (up to 4 hours; see Figure 2) and correlates with recovery times observed in the clinical setting after infusions up to 12 hours. Concentrations of remifentanil are proportional to the dose administered throughout the recommended dose range. The pharmacokinetics of remifentanil are unaffected by the presence of renal or hepatic impairment. | ||
| Line 244: | Line 237: | ||
Remifentanil clearance is not altered by concomitant administration of thiopental, isoflurane, propofol, or temazepam during anesthesia. In vitro studies with atracurium, mivacurium, esmolol, echothiophate, neostigmine, physostigmine, and midazolam revealed no inhibition of remifentanil hydrolysis in whole human blood by these drugs. | Remifentanil clearance is not altered by concomitant administration of thiopental, isoflurane, propofol, or temazepam during anesthesia. In vitro studies with atracurium, mivacurium, esmolol, echothiophate, neostigmine, physostigmine, and midazolam revealed no inhibition of remifentanil hydrolysis in whole human blood by these drugs. | ||
|alcohol=Alcohol-Remifentanil interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Remifentanil interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 21:46, 10 June 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Chetan Lokhande, M.B.B.S [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Remifentanil is an analgesic opioid that is FDA approved for the {{{indicationType}}} of general anesthesia; adjunct, monitored anesthesia care sedation, analgesic component; adjunct, postoperative pain, immediate postoperative period. Common adverse reactions include cardiovascular: hypotension (19% or less ), dermatologic: pruritus (less than 1% to 18% ), gastrointestinal: nausea (less than 1% to 44% ), vomiting (less than 1% to 22% ), musculoskeletal: muscle rigidity (11% or less) , neurologic: headache (18% or less ).
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Exceeding the recommended dose (greater than 1 and up to 20 mcg/kg) or exceeding the recommended infusion rate (starting dose greater than 0.1 mcg/kg/min) results in higher incidence of adverse events.
- Administering bolus doses to spontaneously breathing patients is not recommended.
- Analgesia for a mechanically ventilated patient, ICU: continuous infusion, 0.1 to 0.15 mcg/kg/min IV initially (using ideal body weight), with titration to a max dose of 0.2 to 0.4 mcg/kg/min IV (clinical studies).
- General anesthesia; adjunct: induction, 0.5 to 1 mcg/kg/min IV; 1 mcg/kg IV over 30 to 60 seconds if intubated within 8 minutes of initiation.
- General anesthesia; adjunct: maintenance, 0.25 mcg/kg/min IV (range 0.05 to 2 mcg/kg/min) plus isoflurane or propofol; supplemental bolus of 1 mcg/kg every 2 to 5 minutes if needed.
- General anesthesia; adjunct: maintenance, 0.4 mcg/kg/min IV (range 0.1 to 2 mcg/kg/min IV) plus nitrous oxide; supplemental bolus of 1 mcg/kg every 2 to 5 minutes if needed.
- General anesthesia; adjunct: CABG, during induction through intubation, 1 mcg/kg/min IV.
- General anesthesia; adjunct: CABG, during maintenance of anesthesia, 1 mcg/kg/min IV (range 0.125 to 4 mcg/kg/min); supplemental bolus of 0.5 to 1 mcg/kg if needed.
- Monitored anesthesia care sedation, Analgesic component; adjunct: single dose, used with midazolam, 0.5 mcg/kg IV injection over 30 to 60 seconds as single dose 90 seconds before administration of local anesthetic.
- Monitored anesthesia care sedation, Analgesic component; adjunct: single dose, used alone 1 mcg/kg IV injection over 30 to 60 seconds as single dose 90 seconds before administration of local anesthetic.
- Monitored anesthesia care sedation, Analgesic component; adjunct: continuous infusion, used WITH midazolam, 0.05 mcg/kg/min IV infusion 5 minutes before placement of local or regional block; after placement of block, decrease dose to 0.025 mcg/kg/min (range 0.025 to 0.2 mcg/kg/min), adjust dose in 0.025 mcg/kg/min increments at 5-minute intervals.
- Monitored anesthesia care sedation, Analgesic component; adjunct: continuous infusion, used alone, 0.1 mcg/kg/min IV infusion 5 minutes before placement of local or regional block; after placement of block, decrease dose to 0.05 mcg/kg/min (range 0.025 to 0.2 mcg/kg/min), adjust dose in 0.025 mcg/kg/min increments at 5-minute intervals.
- Postoperative pain, Immediate postoperative period: 0.1 mcg/kg/min IV, adjust infusion every 5 minutes in 0.025 mcg/kg/min increments to reach desired effect (range 0.025 to 0.2 mcg/kg/min) [1]
- Postoperative pain, Immediate postoperative period: CABG, 1 mcg/kg/min IV infusion (range 0.05 to 1 mcg/kg/min).
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
- Analgesia for a mechanically ventilated patient, ICU.
- Procedural sedation.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Remifentanil in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Efficacy for use in pediatric patients during the immediate postoperative period or for use as a component of monitored anesthesia care has not been established.
- Administering bolus doses to spontaneously breathing patients is not recommended.
- Analgesia for a mechanically ventilated patient, ICU: (infants) continuous infusion, 0.075 to 0.15 mcg/kg/min IV initially, with titration to a max dose of 0.5 to 0.94 mcg/kg/min IV (clinical studies).
- Analgesia for a mechanically ventilated patient, ICU: (children) continuous infusion, 0.1 mcg/kg/min IV (clinical study) [9]
- General anesthesia; Adjunct: (age birth to 2 months) maintenance, 0.4 mcg/kg/min IV (range 0.4 to 1 mcg/kg/min) plus nitrous oxide; supplemental bolus of 1 mcg/kg every 2 to 5 minutes if needed.
- General anesthesia; Adjunct: (age 1 year to 12 years) maintenance, 0.25 mcg/kg/min IV (range 0.05 to 1.3 mcg/kg/min) plus halothane, sevoflurane, or isoflurane; supplemental bolus of 1 mcg/kg every 2 to 5 minutes if needed.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information about Off-Label Guideline-Supported Use of Remifentanil in pediatric patients.
Non–Guideline-Supported Use
There is limited information about Off-Label Non–Guideline-Supported Use of Remifentanil in pediatric patients.
Contraindications
Due to the presence of glycine in the formulation, ULTIVA is contraindicated for epidural or intrathecal administration. ULTIVA is also contraindicated in patients with known hypersensitivity to fentanyl analogs.
Warnings
Continuous infusions of ULTIVA should be administered only by an infusion device. IV bolus administration of ULTIVA should be used only during the maintenance of general anesthesia. In nonintubated patients, single doses of ULTIVA should be administered over 30 to 60 seconds.
Interruption of an infusion of ULTIVA will result in rapid offset of effect. Rapid clearance and lack of drug accumulation result in rapid dissipation of respiratory depressant and analgesic effects upon discontinuation of ULTIVA at recommended doses. Discontinuation of an infusion of ULTIVA should be preceded by the establishment of adequate postoperative analgesia.
Injections of ULTIVA should be made into IV tubing at or close to the venous cannula. Upon discontinuation of ULTIVA, the IV tubing should be cleared to prevent the inadvertent administration of ULTIVA at a later point in time. Failure to adequately clear the IV tubing to remove residual ULTIVA has been associated with the appearance of respiratory depression, apnea, and muscle rigidity upon the administration of additional fluids or medications through the same IV tubing.
USE OF ULTIVA IS ASSOCIATED WITH APNEA AND RESPIRATORY DEPRESSION. ULTIVA SHOULD BE ADMINISTERED ONLY BY PERSONS SPECIFICALLY TRAINED IN THE USE OF ANESTHETIC DRUGS AND THE MANAGEMENT OF THE RESPIRATORY EFFECTS OF POTENT OPIOIDS, INCLUDING RESPIRATORY AND CARDIAC RESUSCITATION OF PATIENTS IN THE AGE GROUP BEING TREATED. SUCH TRAINING MUST INCLUDE THE ESTABLISHMENT AND MAINTENANCE OF A PATENT AIRWAY AND ASSISTED VENTILATION.
ULTIVA SHOULD NOT BE USED IN DIAGNOSTIC OR THERAPEUTIC PROCEDURES OUTSIDE THE MONITORED ANESTHESIA CARE SETTING. PATIENTS RECEIVING MONITORED ANESTHESIA CARE SHOULD BE CONTINUOUSLY MONITORED BY PERSONS NOT INVOLVED IN THE CONDUCT OF THE SURGICAL OR DIAGNOSTIC PROCEDURE. OXYGEN SATURATION SHOULD BE MONITORED ON A CONTINUOUS BASIS.
RESUSCITATIVE AND INTUBATION EQUIPMENT, OXYGEN, AND AN OPIOID ANTAGONIST MUST BE READILY AVAILABLE.
Respiratory depression in spontaneously breathing patients is generally managed by decreasing the rate of the infusion of ULTIVA by 50% or by temporarily discontinuing the infusion.
Skeletal muscle rigidity can be caused by ULTIVA and is related to the dose and speed of administration. ULTIVA may cause chest wall rigidity (inability to ventilate) after single doses of >1 mcg/kg administered over 30 to 60 seconds, or after infusion rates >0.1 mcg/kg/min. Single doses <1 mcg/kg may cause chest wall rigidity when given concurrently with a continuous infusion of ULTIVA.
Muscle rigidity induced by ULTIVA should be managed in the context of the patient's clinical condition. Muscle rigidity occurring during the induction of anesthesia should be treated by the administration of a neuromuscular blocking agent and the concurrent induction medications.
Muscle rigidity seen during the use of ULTIVA in spontaneously breathing patients may be treated by stopping or decreasing the rate of administration of ULTIVA. Resolution of muscle rigidity after discontinuing the infusion of ULTIVA occurs within minutes. In the case of life-threatening muscle rigidity, a rapid onset neuromuscular blocker or naloxone may be administered.
ULTIVA should not be administered into the same IV tubing with blood due to potential inactivation by nonspecific esterases in blood products.
Adverse Reactions
Clinical Trials Experience
ULTIVA produces adverse events that are characteristic of µ-opioids, such as respiratory depression, bradycardia, hypotension, and skeletal muscle rigidity. These adverse events dissipate within minutes of discontinuing or decreasing the infusion rate of ULTIVA. See CLINICAL PHARMACOLOGY, WARNINGS, and PRECAUTIONS on the management of these events.
Adverse event information is derived from controlled clinical trials that were conducted in a variety of surgical procedures of varying duration, using a variety of premedications and other anesthetics, and in patient populations with diverse characteristics including underlying disease.
Adults
Approximately 2770 adult patients were exposed to ULTIVA in controlled clinical trials. The frequencies of adverse events during general anesthesia with the recommended doses of ULTIVA are given in Table 3. Each patient was counted once for each type of adverse event.
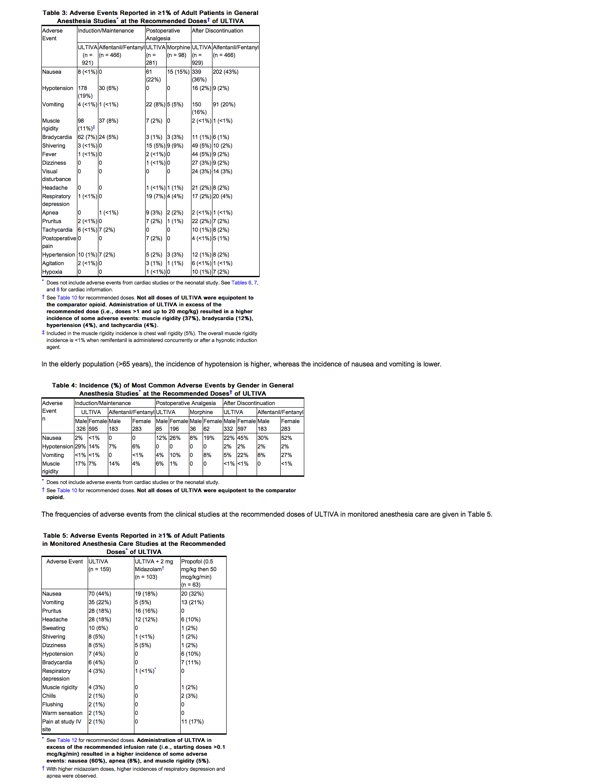
The frequencies of adverse events from the clinical studies at the recommended doses of ULTIVA in monitored anesthesia care are given in Table 5.
Other Adverse Events in Adult Patients
The frequencies of less commonly reported adverse clinical events from all controlled general anesthesia and monitored anesthesia care studies are presented below.
Event frequencies are calculated as the number of patients who were administered ULTIVA and reported an event divided by the total number of patients exposed to ULTIVA in all controlled studies including cardiac dose-ranging and neurosurgery studies (n = 1883 general anesthesia, n = 609 monitored anesthesia care).
Incidence Less than 1%
Digestive: constipation, abdominal discomfort, xerostomia, gastro-esophageal reflux, dysphagia, diarrhea, heartburn, ileus.
Cardiovascular: various atrial and ventricular arrhythmias, heart block, ECG change consistent with myocardial ischemia, elevated CPK-MB level, syncope.
Musculoskeletal: muscle stiffness, musculoskeletal chest pain.
Respiratory: cough, dyspnea, bronchospasm, laryngospasm, rhonchi, stridor, nasal congestion, pharyngitis, pleural effusion, hiccup(s), pulmonary edema, rales, bronchitis, rhinorrhea.
Nervous: anxiety, involuntary movement, prolonged emergence from anesthesia, confusion, awareness under anesthesia without pain, rapid awakening from anesthesia, tremors, disorientation, dysphoria, nightmare(s), hallucinations, paresthesia, nystagmus, twitch, sleep disorder, seizure, amnesia.
Body as a Whole: decreased body temperature, anaphylactic reaction, delayed recovery from neuromuscular block.
Skin: rash, urticaria.
Urogenital: urine retention, oliguria, dysuria, urine incontinence.
Infusion Site Reaction: erythema, pruritus, rash.
Metabolic and Nutrition: abnormal liver function, hyperglycemia, electrolyte disorders, increased CPK level.
Hematologic and Lymphatic: anemia, lymphopenia, leukocytosis, thrombocytopenia.
The frequencies of adverse events from the clinical studies at the recommended doses of ULTIVA in cardiac surgery are given in Tables 6, 7, and 8. These tables represent adverse events collected during discrete phases of cardiac surgery. Any event should be viewed as temporally associated with drug administration and the phase indicated should not be perceived as the only time the event might occur.
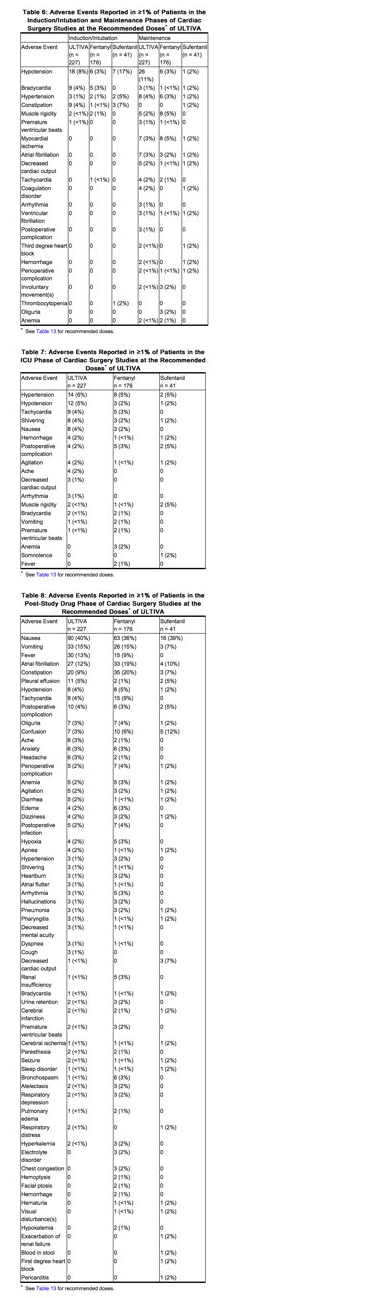
Pediatrics
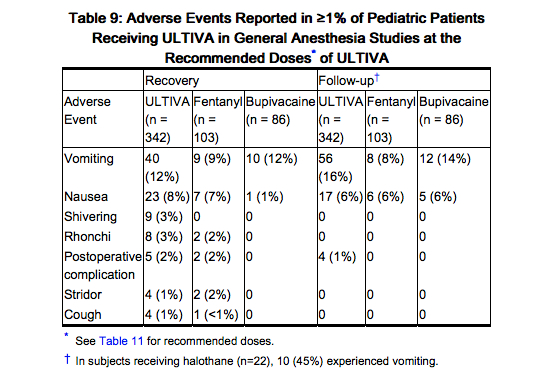
ULTIVA has been studied in 342 pediatric patients in controlled clinical trials for maintenance of general anesthesia. In the pediatric population (birth to 12 years), the most commonly reported events were nausea, vomiting, and shivering.
The frequencies of adverse events during general anesthesia with the recommended doses of ULTIVA are given in Table 9. Each patient was counted once for each type of adverse event. There were no adverse events ≥1% for any treatment group during the maintenance period in the pediatric patient general anesthesia studies.
Observed During Clinical Practice
In addition to adverse events reported from clinical trials, the following events have been identified during post-approval use of remifentanil in conjunction with one or more anesthetic agents in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to remifentanil.
Cardiovascular: Asystole.
Non-Site Specific: Anaphylactic/anaphylactoid responses, which in some cases have been severe (e.g., shock).
Postmarketing Experience
There is limited information regarding Remifentanil Postmarketing Experience in the drug label.
Drug Interactions
There is limited information regarding Remifentanil Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA):
There is no FDA guidance on usage of Remifentanil in women who are pregnant.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Remifentanil in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Remifentanil during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Remifentanil in women who are nursing.
Pediatric Use
There is no FDA guidance on the use of Remifentanil in pediatric settings.
Geriatic Use
There is no FDA guidance on the use of Remifentanil in geriatric settings.
Gender
There is no FDA guidance on the use of Remifentanil with respect to specific gender populations.
Race
There is no FDA guidance on the use of Remifentanil with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Remifentanil in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Remifentanil in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Remifentanil in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Remifentanil in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Remifentanil Administration in the drug label.
Monitoring
There is limited information regarding Remifentanil Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Remifentanil and IV administrations.
Overdosage
There is limited information regarding Remifentanil overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
There is limited information regarding Remifentanil Pharmacology in the drug label.
Mechanism of Action
ULTIVA is a µ-opioid agonist with rapid onset and peak effect, and short duration of action. The µ-opioid activity of ULTIVA is antagonized by opioid antagonists such as naloxone.
Unlike other opioids, ULTIVA is rapidly metabolized by hydrolysis of the propanoic acid-methyl ester linkage by nonspecific blood and tissue esterases. ULTIVA is not a substrate for plasma cholinesterase (pseudocholinesterase) and, therefore, patients with atypical cholinesterase are expected to have a normal duration of action.
Structure
ULTIVA (remifentanil hydrochloride) for Injection is a µ-opioid agonist chemically designated as a 3-[4-methoxycarbonyl-4-[(1-oxopropyl)phenylamino]-1-piperidine]propanoic acid methyl ester, hydrochloride salt, C20H28N2O5•HCl, with a molecular weight of 412.91. It has the following chemical structure:
ULTIVA is a sterile, nonpyrogenic, preservative-free, white to off-white lyophilized powder for intravenous (IV) administration after reconstitution and dilution. Each vial contains 1, 2, or 5 mg of remifentanil base; 15 mg glycine; and hydrochloric acid to buffer the solutions to a nominal pH of 3 after reconstitution. When reconstituted as directed, solutions of ULTIVA are clear and colorless and contain remifentanil hydrochloride (HCl) equivalent to 1 mg/mL of remifentanil base. The pH of reconstituted solutions of ULTIVA ranges from 2.5 to 3.5. Remifentanil HCl has a pKa of 7.07. Remifentanil HCl has an n-octanol:water partition coefficient of 17.9 at pH 7.3.
Pharmacodynamics
The analgesic effects of ULTIVA are rapid in onset and offset. Its effects and side effects are dose dependent and similar to other µ-opioids. ULTIVA in humans has a rapid blood-brain equilibration half-time of 1 ± 1 minutes (mean ± SD) and a rapid onset of action. The pharmacodynamic effects of ULTIVA closely follow the measured blood concentrations, allowing direct correlation between dose, blood levels, and response. Blood concentration decreases 50% in 3 to 6 minutes after a 1-minute infusion or after prolonged continuous infusion due to rapid distribution and elimination processes and is independent of duration of drug administration. Recovery from the effects of ULTIVA occurs rapidly (within 5 to 10 minutes). New steady-state concentrations occur within 5 to 10 minutes after changes in infusion rate. When used as a component of an anesthetic technique, ULTIVA can be rapidly titrated to the desired depth of anesthesia/analgesia (e.g., as required by varying levels of intraoperative stress) by changing the continuous infusion rate or by administering an IV bolus injection.
Hemodynamics
In premedicated patients undergoing anesthesia, 1-minute infusions of <2 mcg/kg of ULTIVA cause dose-dependent hypotension and bradycardia. While additional doses >2 mcg/kg (up to 30 mcg/kg) do not produce any further decreases in heart rate or blood pressure, the duration of the hemodynamic change is increased in proportion to the blood concentrations achieved. Peak hemodynamic effects occur within 3 to 5 minutes of a single dose of ULTIVA or an infusion rate increase. Glycopyrrolate, atropine, and vagolytic neuromuscular blocking agents attenuate the hemodynamic effects associated with ULTIVA. When appropriate, bradycardia and hypotension can be reversed by reduction of the rate of infusion of ULTIVA, or the dose of concurrent anesthetics, or by the administration of fluids or vasopressors.
Respiration
ULTIVA depresses respiration in a dose-related fashion. Unlike other fentanyl analogs, the duration of action of ULTIVA at a given dose does not increase with increasing duration of administration, due to lack of drug accumulation. When ULTIVA and alfentanil were dosed to equal levels of respiratory depression, recovery of respiratory drive after 3-hour infusions was more rapid and less variable with ULTIVA (see Figure 1).
pontaneous respiration occurs at blood concentrations of 4 to 5 ng/mL in the absence of other anesthetic agents; for example, after discontinuation of a 0.25-mcg/kg/min infusion of remifentanil, these blood concentrations would be reached in 2 to 4 minutes. In patients undergoing general anesthesia, the rate of respiratory recovery depends upon the concurrent anesthetic; N2O < propofol < isoflurane (see CLINICAL TRIALS: Recovery).
Muscle Rigidity
Skeletal muscle rigidity can be caused by ULTIVA and is related to the dose and speed of administration. ULTIVA may cause chest wall rigidity (inability to ventilate) after single doses of >1 mcg/kg administered over 30 to 60 seconds or infusion rates >0.1 mcg/kg/min; peripheral muscle rigidity may occur at lower doses. Administration of doses <1 mcg/kg may cause chest wall rigidity when given concurrently with a continuous infusion of ULTIVA. Prior or concurrent administration of a hypnotic (propofol or thiopental) or a neuromuscular blocking agent may attenuate the development of muscle rigidity. Excessive muscle rigidity can be treated by decreasing the rate or discontinuing the infusion of ULTIVA or by administering a neuromuscular blocking agent.
Histamine Release
Assays of histamine in patients and normal volunteers have shown no elevation in plasma histamine levels after administration of ULTIVA in doses up to 30 mcg/kg over 60 seconds.
Analgesia
Infusions of 0.05 to 0.1 mcg/kg/min, producing blood concentrations of 1 to 3 ng/mL, are typically associated with analgesia with minimal decrease in respiratory rate. Supplemental doses of 0.5 to 1 mcg/kg, incremental increases in infusion rate >0.05 mcg/kg/min, and blood concentrations exceeding 5 ng/mL (typically produced by infusions of 0.2 mcg/kg/min) have been associated with transient and reversible respiratory depression, apnea, and muscle rigidity.
Anesthesia
ULTIVA is synergistic with the activity of hypnotics (propofol and thiopental), inhaled anesthetics, and benzodiazepines (see CLINICAL TRIALS, PRECAUTIONS, and DOSAGE AND ADMINISTRATION).
Age
The pharmacodynamic activity of ULTIVA (as measured by the EC50 for development of delta waves on the EEG) increases with increasing age. The EC50 of remifentanil for this measure was 50% less in patients over 65 years of age when compared to healthy volunteers (25 years of age) (see DOSAGE AND ADMINISTRATION).
Gender
No differences have been shown in the pharmacodynamic activity (as measured by the EEG) of ULTIVA between men and women.
Drug Interactions
In animals the duration of muscle paralysis from succinylcholine is not prolonged by remifentanil.
Intraocular Pressure
There was no change in intraocular pressure after the administration of ULTIVA prior to ophthalmic surgery under monitored anesthesia care.
Cerebrodynamics
Under isoflurane-nitrous oxide anesthesia (PaCO2 <30 mmHg), a 1-minute infusion of ULTIVA (0.5 or 1.0 mcg/kg) produced no change in intracranial pressure. Mean arterial pressure and cerebral perfusion decreased as expected with opioids. In patients receiving ULTIVA and nitrous oxide anesthesia, cerebrovascular reactivity to carbon dioxide remained intact. In humans, no epileptiform activity was seen on the EEG (n = 44) at remifentanil doses up to 8 mcg/kg/min.
Renal Dysfunction
The pharmacodynamics of ULTIVA (ventilatory response to hypercarbia) are unaltered in patients with end stage renal disease (creatinine clearance <10 mL/min).
Hepatic Dysfunction
The pharmacodynamics of ULTIVA (ventilatory response to hypercarbia) are unaltered in patients with severe hepatic dysfunction awaiting liver transplant.
Pharmacokinetics
After IV doses administered over 60 seconds, the pharmacokinetics of remifentanil fit a three-compartment model with a rapid distribution half-life of 1 minute, a slower distribution half-life of 6 minutes, and a terminal elimination half-life of 10 to 20 minutes. Since the terminal elimination component contributes less than 10% of the overall area under the concentration versus time curve (AUC), the effective biological half-life of ULTIVA is 3 to 10 minutes. This is similar to the 3- to 10-minute half-life measured after termination of prolonged infusions (up to 4 hours; see Figure 2) and correlates with recovery times observed in the clinical setting after infusions up to 12 hours. Concentrations of remifentanil are proportional to the dose administered throughout the recommended dose range. The pharmacokinetics of remifentanil are unaffected by the presence of renal or hepatic impairment.
Distribution
The initial volume of distribution (Vd) of remifentanil is approximately 100 mL/kg and represents distribution throughout the blood and rapidly perfused tissues. Remifentanil subsequently distributes into peripheral tissues with a steady-state volume of distribution of approximately 350 mL/kg. These two distribution volumes generally correlate with total body weight (except in severely obese patients when they correlate better with ideal body weight [IBW]). Remifentanil is approximately 70% bound to plasma proteins of which two-thirds is binding to alpha-1-acid-glycoprotein.
Metabolism
Remifentanil is an esterase-metabolized opioid. A labile ester linkage renders this compound susceptible to hydrolysis by nonspecific esterases in blood and tissues. This hydrolysis results in the production of the carboxylic acid metabolite (3-[4-methoxycarbonyl-4-[(1-oxopropyl)phenylamino]-1-piperidine]propanoic acid), and represents the principal metabolic pathway for remifentanil (>95%). The carboxylic acid metabolite is essentially inactive (1/4600 as potent as remifentanil in dogs) and is excreted by the kidneys with an elimination half-life of approximately 90 minutes. Remifentanil is not metabolized by plasma cholinesterase (pseudocholinesterase) and is not appreciably metabolized by the liver or lung.
Elimination
The clearance of remifentanil in young, healthy adults is approximately 40 mL/min/kg. Clearance generally correlates with total body weight (except in severely obese patients when it correlates better with IBW). The high clearance of remifentanil combined with a relatively small volume of distribution produces a short elimination half-life of approximately 3 to 10 minutes (see Figure 2). This value is consistent with the time taken for blood or effect site concentrations to fall by 50% (context-sensitive half-times) which is approximately 3 to 6 minutes. Unlike other fentanyl analogs, the duration of action does not increase with prolonged administration.
Titration to Effect
The rapid elimination of remifentanil permits the titration of infusion rate without concern for prolonged duration. In general, every 0.1-mcg/kg/min change in the IV infusion rate will lead to a corresponding 2.5-ng/mL change in blood remifentanil concentration within 5 to 10 minutes. In intubated patients only, a more rapid increase (within 3 to 5 minutes) to a new steady state can be achieved with a 1.0-mcg/kg bolus dose in conjunction with an infusion rate increase.
Special Populations
Pediatrics
In pediatric patients, 5 days to 17 years of age (n = 47), the clearance and volume of distribution of remifentanil were increased in younger children and declined to young healthy adult values by age 17. The average clearance of remifentanil in neonates (less than 2 months of age) was approximately 90.5 ± 36.8 mL/min/kg (mean ± SD) while in adolescents (13 to 16 years) this value was 57.2 ± 21.1 mL/min/kg. The total (steady-state) volume of distribution in neonates was 452 ± 144 mL/kg versus 223 ± 30.6 mL/kg in adolescents. The half-life of remifentanil was the same in neonates and adolescents. Clearance of remifentanil was maintained at or above normal adult values in patients 5 days to 17 years of age.
Renal Impairment
The pharmacokinetic profile of ULTIVA is not changed in patients with end stage renal disease (creatinine clearance <10 mL/min). In anephric patients, the half-life of the carboxylic acid metabolite increases from 90 minutes to 30 hours. The metabolite is removed by hemodialysis with a dialysis extraction ratio of approximately 30%.
Hepatic Impairment
The pharmacokinetics of remifentanil and its carboxylic acid metabolite are unchanged in patients with severe hepatic impairment.
Elderly
The clearance of remifentanil is reduced (approximately 25%) in the elderly (>65 years of age) compared to young adults (average 25 years of age). However, remifentanil blood concentrations fall as rapidly after termination of administration in the elderly as in young adults.
Gender
There is no significant difference in the pharmacokinetics of remifentanil in male and female patients after correcting for differences in weight.
Obesity
There is no difference in the pharmacokinetics of remifentanil in non-obese versus obese (greater than 30% over IBW) patients when normalized to IBW.
Cardiopulmonary Bypass (CPB)
Remifentanil clearance is reduced by approximately 20% during hypothermic CPB.
Drug Interactions
Remifentanil clearance is not altered by concomitant administration of thiopental, isoflurane, propofol, or temazepam during anesthesia. In vitro studies with atracurium, mivacurium, esmolol, echothiophate, neostigmine, physostigmine, and midazolam revealed no inhibition of remifentanil hydrolysis in whole human blood by these drugs.
Nonclinical Toxicology
There is limited information regarding Remifentanil Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Remifentanil Clinical Studies in the drug label.
How Supplied
There is limited information regarding Remifentanil How Supplied in the drug label.
Storage
There is limited information regarding Remifentanil Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Remifentanil |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Remifentanil |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Remifentanil Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Remifentanil interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Remifentanil Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Remifentanil Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.