Radiation injury
| Radiation injury | |
 | |
|---|---|
| Radiation Hazard symbol. | |
| ICD-10 | T66 |
| ICD-9 | 990 |
|
WikiDoc Resources for Radiation injury |
|
Articles |
|---|
|
Most recent articles on Radiation injury Most cited articles on Radiation injury |
|
Media |
|
Powerpoint slides on Radiation injury |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Radiation injury at Clinical Trials.gov Trial results on Radiation injury Clinical Trials on Radiation injury at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Radiation injury NICE Guidance on Radiation injury
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Radiation injury Discussion groups on Radiation injury Patient Handouts on Radiation injury Directions to Hospitals Treating Radiation injury Risk calculators and risk factors for Radiation injury
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Radiation injury |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Radiation poisoning, also called "radiation sickness", is a form of damage to organ tissue due to excessive exposure to ionizing radiation. The term is generally used to refer to acute problems caused by a large dosage of radiation in a short period. Many of the symptoms of radiation poisoning occur as ionizing radiation interferes with cell division. This interference allows for treatment of cancer cells; such cells are among the fastest-dividing in the body, and may be destroyed by a radiation dose that adjacent normal cells are likely to survive.
The clinical name for "radiation sickness" is acute radiation syndrome as described by the CDC[4][5][6][7]. A chronic radiation syndrome does exist but is very uncommon; this has been observed among workers in early radium source production sites and in the early days of the Soviet nuclear program. A short exposure can result in acute radiation syndrome; chronic radiation syndrome requires a prolonged high level of exposure.
Measuring radiation dosage
The rad is a unit of absorbed radiation dose defined in terms of the energy actually deposited in the tissue. One rad is an absorbed dose of 0.01 joules of energy per kilogram of tissue. The more recent SI unit is the gray (Gy), which is defined as 1 joule of deposited energy per kilogram of tissue. Thus one gray is equal to 100 rad.
To accurately assess the risk of radiation, the absorbed dose energy in rad is multiplied by the relative biological effectiveness (RBE) of the radiation to get the biological dose equivalent in rems. Rem stands for "Röntgen equivalent in man (sic)." In SI units, the absorbed dose energy in grays is multiplied by the same RBE to get a biological dose equivalent in sieverts (Sv). The sievert is equal to 100 rem.
The RBE is a "quality factor," often denoted by the letter Q, which assesses the damage to tissue caused by a particular type and energy of radiation. For alpha particles Q may be as high as 20, so that one rad of alpha radiation is equivalent to 20 rem. The Q of neutron radiation depends on their energy. However, for beta particles, x-rays, and gamma rays, Q is taken as one, so that the rad and rem are equivalent for those radiation sources, as are the gray and sievert. See the sievert article for a more complete list of Q values.
Table of exposure levels and symptoms
Dose-equivalents are presently stated in sieverts:
0.05–0.2 Sv (5–20 REM)
No symptoms. Potential for cancer and mutation of genetic material, according to the Linear no threshold model (LNT model): this is disputed (Note: see hormesis). A few researchers contend that low dose radiation may be beneficial. [8] [9] [10] 50 mSv is the yearly federal limit for radiation workers in the United States. In the UK the yearly limit for a classified radiation worker is 20 mSv. In Canada, the single-year maximum is 50 mSv, but the maximum 5-year dose is only 100 mSv. Company limits are usually stricter so as not to violate federal limits. [11]
0.2–0.5 Sv (20–50 REM)
No noticeable symptoms. Red blood cell count decreases temporarily.
0.5–1 Sv (50–100 REM)
Mild radiation sickness with headache and increased risk of infection due to disruption of immunity cells. Temporary male sterility is possible.
1–2 Sv (100–200 REM)
Light radiation poisoning, 10% fatality after 30 days (LD 10/30). Typical symptoms include mild to moderate nausea (50% probability at 2 Sv), with occasional vomiting, beginning 3 to 6 hours after irradiation and lasting for up to one day. This is followed by a 10 to 14 day latent phase, after which light symptoms like general illness and fatigue appear (50% probability at 2 Sv). The immune system is depressed, with convalescence extended and increased risk of infection. Temporary male sterility is common. Spontaneous abortion or stillbirth will occur in pregnant women.
2–3 Sv (200–300 REM)
Moderate radiation poisoning, 35% fatality after 30 days (LD 35/30). Nausea is common (100% at 3 Sv), with 50% risk of vomiting at 2.8 Sv. Symptoms onset at 1 to 6 hours after irradiation and last for 1 to 2 days. After that, there is a 7 to 14 day latent phase, after which the following symptoms appear: loss of hair all over the body (50% probability at 3 Sv), fatigue and general illness. There is a massive loss of leukocytes (white blood cells), greatly increasing the risk of infection. Permanent female sterility is possible. Convalescence takes one to several months.
3–4 Sv (300–400 REM)
Severe radiation poisoning, 50% fatality after 30 days (LD 50/30). Other symptoms are similar to the 2–3 Sv dose, with uncontrollable bleeding in the mouth, under the skin and in the kidneys (50% probability at 4 Sv) after the latent phase.
4–6 Sv (400–600 REM)
Acute radiation poisoning, 60% fatality after 30 days (LD 60/30). Fatality increases from 60% at 4.5 Sv to 90% at 6 Sv (unless there is intense medical care). Symptoms start half an hour to two hours after irradiation and last for up to 2 days. After that, there is a 7 to 14 day latent phase, after which generally the same symptoms appear as with 3-4 Sv irradiation, with increased intensity. Female sterility is common at this point. Convalescence takes several months to a year. The primary causes of death (in general 2 to 12 weeks after irradiation) are infections and internal bleeding.
6–10 Sv (600–1,000 REM)
Acute radiation poisoning, near 100% fatality after 14 days (LD 100/14). Survival depends on intense medical care. Bone marrow is nearly or completely destroyed, so a bone marrow transplant is required. Gastric and intestinal tissue are severely damaged. Symptoms start 15 to 30 minutes after irradiation and last for up to 2 days. Subsequently, there is a 5 to 10 day latent phase, after which the person dies of infection or internal bleeding. Recovery would take several years and probably would never be complete.
Devair Alves Ferreira received a dose of approximately 7.0 Sv (700 REM) during the Goiânia accident and survived, partially due to his fractionated exposure.
10–50 Sv (1,000–5,000 REM)
Acute radiation poisoning, 100% fatality after 7 days (LD 100/7). An exposure this high leads to spontaneous symptoms after 5 to 30 minutes. After powerful fatigue and immediate nausea caused by direct activation of chemical receptors in the brain by the irradiation, there is a period of several days of comparative well-being, called the latent (or "walking ghost") phase. After that, cell death in the gastric and intestinal tissue, causing massive diarrhea, intestinal bleeding and loss of water, leads to water-electrolyte imbalance. Death sets in with delirium and coma due to breakdown of circulation. Death is currently inevitable; the only treatment that can be offered is pain therapy.
Louis Slotin was exposed to approximately 21 Sv in a critical accident on 21 May 1946, and died nine days later on 30 May.
At this dose the skin can be damaged. Here is a photo of a man who received a 10 to 20 Gy gamma whole body dose as a result of an industrial accident. He died about 10 days after the photo was taken, about 30 days after the event.
More than 50 Sv (>5,000 REM)
A worker receiving 100 Sv (10,000 REM) in an accident at Wood River, Rhode Island, USA on 24 July 1964 survived for 49 hours after exposure, and an operator receiving between 60 and 180 Sv (18,000 REM) to his upper body in an accident at Los Alamos, New Mexico, USA on 30 December 1958 survived for 36 hours; details of this accident can be found on page 16 (page 30 in the PDF version) of Los Alamos' 2000 Review of Criticality Accidents [12].
Acute (short-term) vs chronic (long-term) effects
Radiation sickness is generally associated with acute exposure and has a characteristic set of symptoms that appear in an orderly fashion. The symptoms of radiation sickness become more serious (and the chance of survival decreases) as the dosage of radiation increases. These effects are described as the deterministic effects of radiation.
Longer term exposure to radiation, at doses less than that which produces serious radiation sickness, can induce cancer as cell-cycle genes are mutated. If a cancer is radiation-induced, then the disease, the speed at which the condition advances, the prognosis, the degree of pain, and every other feature of the disease are not functions of the radiation dose to which the sufferer is exposed.
Since tumors grow by abnormally rapid cell division, the ability of radiation to disturb cell division is also used to treat cancer (see radiotherapy), and low levels of ionizing radiation have been claimed to lower one's risk of cancer (see hormesis).
Exposure
External vs internal exposure
External
External exposure is exposure which occurs when the radioactive source (or other radiation source) is outside (and remains outside) the organism which is exposed. Below are a series of three examples of external exposure.
- A person who places a sealed radioactive source in their pocket
- A space traveller who is irradiated by cosmic rays
- A person who is treated for cancer by either teletherapy or brachytherapy. While in brachytherapy the source is inside the person it is still external exposure because the active part of the source never comes into direct contact with the biological tissues of the person.
One of the key points is that external exposure is often relatively easy to estimate, and if the irradiated objects do not become radioactive (except for a case where the radiation is an intense neutron beam which causes activation of the object'). It is possible for an object to be contaminated on the outer surfaces, assuming that no radioactivity enters the object it is still a case of external exposure and it is normally the case that decontamination is easy (wash the surface).
Internal
Internal exposure is when the radioactive material enters the organism, and the radioactive atoms become incorporated into the organism. Below are a series of examples of internal exposure.
- The exposure due to Isotopes of potassium-40K (40K) present within a normal person.
- The exposure to the ingestion of a soluble radioactive substance, such as Strontium-90 (90Sr) in cow’s milk.
- A person who is being treated for cancer by means of an open source radiotherapy method where a radioisotope is used as a drug. A review of this topic was published in 1999.[1]
Because the radioactive material becomes intimately mixed with the affected object it is often difficult to decontaminate the object or person in a case where internal exposure is occurring. While some very insoluble materials such as fission products within a uranium dioxide matrix might never be able to truly become part of an organism, it is normal to consider such particles in the lungs as a form of internal contamination which results in internal exposure. The reasoning is that the particles have entered via an orifice and can not be removed with ease from what the lay person (non biologist) would regard as within the animal. It is important to note that strictly speaking the contents of the digestive tract and the air within the lungs are outside the body of a mammal.
Nuclear warfare
Nuclear warfare is made more complex by virtue of the fact that a person can be irradiated by at least three processes. The first (the major cause of burns) is not caused by ionizing radiation.
- Thermal burns from infrared heat radiation.
- Beta burns from shallow ionizing radiation (this would be from fallout particles, the largest particles in local fallout would be likely to have very high activities due to the fact that they would be deposited so soon after detonation and it is likely that one such particle upon the skin would be able to cause a localized burn), however these particles are very weakly penetrating and have a short range.
- Gamma burns from highly penetrating radiation, this would be likely to cause deep gamma penetration within the body which would result in uniform whole body irradiation rather than only a surface burn. In cases of whole body gamma irradiation (circa 10 Gy) due to accidents involving medical product irradiators some of the human subjects have developed injuries to their skin between the time of irradiation and death.
In the picture on the right the normal clothing that the woman was wearing would have been unable to attenuate the gamma radiation and it is likely that any such effect was evenly applied to her entire body. Beta burns would be likely all over the body due to contact with fallout, but thermal burns are often on one side of the body as heat radiation does not penetrate the human body. In addition, the pattern on her clothing has been burnt into the skin. This is due to the fact that white fabric reflects more infra-red light than dark fabric. As a result the skin close to dark fabric is burnt more than the skin covered by white clothing.
In addition, there is the risk of internal radiation poisoning by ingestion of fallout particles.
Radiation work e.g. industrial radiography
Radiation poisoning can result from accidental exposure to industrial radiation sources. People working with radioactive materials often wear dosimeters or film "badges" to monitor their total exposure to radiation. These devices are more useful than Geiger counters for determining biological effects, as they measure cumulative exposure over time, and are calibrated to change color or otherwise signal the user before exposure reaches unsafe levels. However, film badge types require the film to be developed, as with photographic film, and are used to measure long-term exposure where brief catastrophic exposures are not expected.
Nuclear reactor accidents
Radiation poisoning was a major concern after the Chernobyl reactor accident. It is important to note that in humans the acute effects were largely confined to the accident site. Thirty-one people died as an immediate result.
Of the 100 million curies (4 exabecquerels) of radioactive material, the short lived radioactive isotopes such as 131I Chernobyl released were initially the most dangerous. Due to their short half-lives of 5 and 8 days they have now decayed, leaving the more long-lived caesium-137 (137Cs (with a half-life of 30.07 years) and strontium-90 (90Sr (with a half-life of 28.78 years) as main dangers.
Other accidents
Improper handling of radioactive and nuclear materials lead to radiation release and radiation poisoning. The most serious of these, due to improper disposal of a medical device containing a radioactive source (teletherapy), occurred in Goiânia, Brazil in 1987. It is noteworthy that while the majority of accidents involve smaller industrial radioactive sources (typically used for radiography) a large number of the deaths which have occurred have been due to exposure to the larger sources used for medical purposes.
Ingestion and inhalation
When radioactive compounds enter the human body, the effects are different from those resulting from exposure to an external radiation source. Especially in the case of alpha radiation, which normally does not penetrate the skin, the exposure can be much more damaging after ingestion or inhalation. The radiation exposure is normally expressed as a committed effective dose equivalent (CEDE).
Deliberate poisoning
On November 23, 2006, Alexander Litvinenko died due to suspected deliberate poisoning with polonium-210. His is the first case of confirmed death due to such a cause, although it is also known that there have been other cases where radioactive thallium was used. In addition, an incident occurred in 1990 at Point Lepreau Nuclear Generating Station where several employees acquired small doses of radiation due to the contamination of water in the office watercooler with tritium contaminated heavy water.
Prevention
The best prevention for radiation sickness is to minimize the dose suffered by the human, or to reduce the dose rate.
Time
The longer that the humans are subjected to radiation the larger the dose will be. The advice in the nuclear war manual entitled "Nuclear War Survival Skills" published by Cresson Kearny in the U.S. was that if one needed to leave the shelter then this should be done as rapidly as possible to minimize exposure.
In chapter 12 he states that "Quickly putting or dumping wastes outside is not hazardous once fallout is no longer being deposited. For example, assume the shelter is in an area of heavy fallout and the dose rate outside is 400 R/hr enough to give a potentially fatal dose in about an hour to a person exposed in the open. If a person needs to be exposed for only 10 seconds to dump a bucket, in this 1/360th of an hour he will receive a dose of only about 1 R. Under war conditions, an additional 1-R dose is of little concern."
In peacetime radiation workers are taught to work as quickly as possible when performing a task which exposes them to irradiation. For instance, the recovery of a lost radiography source should be done as quickly as possible.
- <math> \text{Dose} \propto t </math>
Distance
The radiation due to any point source will obey the inverse square law: by doubling the distance the dose rate is quartered. This is why radiation workers are always taught to pick up a gamma source with a pair of tongs rather than their hand.
- <math> \text{Dose} \propto \frac{1}{r^2} </math>
Shielding
By placing a layer of a material which will absorb the radiation between the source and the human, the dose and dose rate can be reduced. For instance, in the event of a nuclear war, it would be a good idea to shelter within a building with thick stone walls (Fallout shelter). During the height of the cold war, fallout shelters were identified in many urban areas. It is interesting to note that, under some conditions, shielding can increase the dose rate. For instance, if the electrons from a high energy beta source (such as 32P) strike a lead surface, X-ray photons will be generated (radiation produced in this way is known as bremsstrahlung). It is best for this reason to cover any high Z materials (such as lead or tungsten) with a low Z material such as aluminium, wood, plastic. This effect can be significant if a person wearing lead-containing gloves picks up a strong beta source. Also, gamma photons can induce the emission of electrons from very dense materials by the photoelectric effect; again, by covering the high Z material with a low Z material, this potential additional source of exposure to humans can be avoided. Furthermore, gamma rays can scatter off a dense object; this enables gamma rays to "go around corners" to a small degree. Hence, to obtain a very high protection factor, the path in/out of the shielded enclosure should have several right angle (90 degree) turns rather than just one.
Reduction of incorporation into the human body
Potassium iodide (KI), administered orally immediately after exposure, may be used to protect the thyroid from ingested radioactive iodine in the event of an accident or terrorist attack at a nuclear power plant, or the detonation of a nuclear explosive. KI would not be effective against a dirty bomb unless the bomb happened to contain radioactive iodine, and even then it would only help to prevent thyroid cancer.
Fractionation of dose
While Devair Alves Ferreira received a large dose during the Goiânia accident of 7.0 Gy, he lived while his wife received a dose of 5.7 Gy and died. The most likely explanation is that his dose was fractionated into many smaller doses which were absorbed over a length of time, while his wife stayed in the house more and was subjected to continuous irradiation without a break, giving her body less time to repair some of the damage done by the radiation. In the same way, some of the people who worked in the basement of the wrecked Chernobyl plant received doses of 10 Gy, but in small fractions, so the acute effects were avoided.
It has been found in radiation biology experiments that if a group of cells are irradiated, then as the dose increases, the number of cells which survive decreases. It has also been found that if a population of cells is given a dose before being set aside (without being irradiated) for a length of time before being irradiated again, then the radiation causes less cell death. The human body contains many types of cells and the human can be killed by the loss of a single type of cells in a vital organ. For many short term radiation deaths (3 days to 30 days), the loss of cells forming blood cells (bone marrow) and the cells in the digestive system (microvilli which form part of the wall of the intestines are constantly being regenerated in a healthy human) causes death.
Cutaneous Radiation Injury
Injury to the skin and underlying tissues from acute exposure to a large external dose of radiation is referred to as cutaneous radiation injury (CRI). Acute radiation syndrome (ARS) 1 will usually be accompanied by some skin damage; however, CRI can occur without symptoms of ARS. This is especially true with acute exposures to beta radiation or low-energy x-rays, because beta radiation and low-energy x-rays are less penetrating and less likely to damage internal organs than gamma radiation is. CRI can occur with radiation doses as low as 2 Gray (Gy) or 200 rads 2 and the severity of CRI symptoms will increase with increasing doses. Most cases of CRI have occurred when people inadvertently came in contact with unsecured radiation sources from food irradiators, radiotherapy equipment, or well depth gauges. In addition, cases of CRI have occurred in people who were overexposed to x-radiation from fluoroscopy units.
Early signs and symptoms of CRI are itching, tingling, or a transient erythema or edema without a history of exposure to heat or caustic chemicals. Exposure to radiation can damage the basal cell layer of the skin and result in inflammation, erythema, and dry or moist desquamation. In addition, radiation damage to hair follicles can cause epilation. Transient and inconsistent erythema (associated with itching) can occur within a few hours of exposure and be followed by a latent, symptom-free phase lasting from a few days to several weeks. After the latent phase, intense reddening, blistering, and ulceration of the irradiated site are visible. Depending on the radiation dose, a third and even fourth wave of erythema are possible over the ensuing months or possibly years.
In most cases, healing occurs by regenerative means; however, large radiation doses to the skin can cause permanent hair loss, damaged sebaceous and sweat glands, atrophy, fibrosis, decreased or increased skin pigmentation, and ulceration or necrosis of the exposed tissue.
With CRI, it is important to keep the following things in mind:
- The visible skin effects depend on the magnitude of the dose as well as the depth of penetration of the radiation.
- Unlike the skin lesions caused by chemical or thermal damage, the lesions caused by radiation exposures do not appear for hours to days following exposure, and burns and other skin effects tend to appear in cycles.
- The key treatment issues with CRI are infection and pain management.
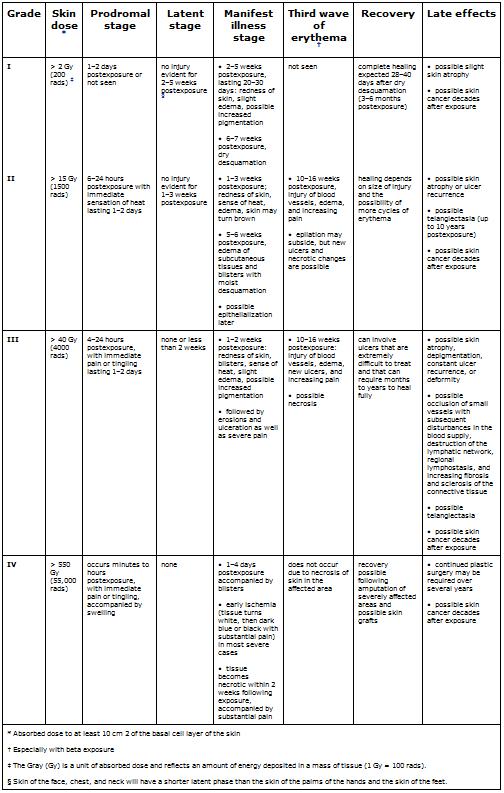
Stages and Grades of CRI
CRI will progress over time in stages and can be categorized by grade, with characteristics of the stages varying by grade of injury, as shown in Table 1. Appendix A gives a detailed description of the various skin responses to radiation, and Appendix B provides color photographs of examples of some of these responses.
Prodromal stage (within hours of exposure)—This stage is characterized by early erythema (first wave of erythema), heat sensations, and itching that define the exposure area. The duration of this stage is from 1 to 2 days.
Latent stage (1–2 days postexposure)—No injury is evident. Depending on the body part, the larger the dose, the shorter this period will last. The skin of the face, chest, and neck will have a shorter latent stage than will the skin of the palms of the hands or the soles of the feet.
Manifest illness stage (days to weeks postexposure)—The basal layer is repopulated through proliferation of surviving clonogenic cells. This stage begins with main erythema (second wave), a sense of heat, and slight edema, which are often accompanied by increased pigmentation. The symptoms that follow vary from dry desquamation or ulceration to necrosis, depending on the severity of the CRI (see Table 1).
Third wave of erythema (10–16 weeks postexposure, especially after beta exposure)—The exposed person experiences late erythema, injury to blood vessels, edema, and increasing pain. A distinct bluish color of the skin can be observed. Epilation may subside, but new ulcers, dermal necrosis, and dermal atrophy (and thinning of the dermis layer) are possible.
Late effects (months to years postexposure; threshold dose ~10 Gy or 1000 rads)—Symptoms can vary from slight dermal atrophy (or thinning of dermis layer) to constant ulcer recurrence, dermal necrosis, and deformity. Possible effects include occlusion of small blood vessels with subsequent disturbances in the blood supply (telangiectasia); destruction of the lymphatic network; regional lymphostasis; and increasing invasive fibrosis, keratosis, vasculitis, and subcutaneous sclerosis of the connective tissue. Pigmentary changes and pain are often present. Skin cancer is possible in subsequent years.
Recovery (months to years)
Patient Management
Diagnosis
The signs and symptoms of CRI are as follows:
- Intensely painful burn-like skin injuries (including itching, tingling, erythema, or edema) without a history of exposure to heat or caustic chemicals
- Note : Erythema will not be seen for hours to days following exposure, and its appearance is cyclic.
- Epilation
- A tendency to bleed
- Possible signs and symptoms of ARS
As mentioned previously, local injuries to the skin from acute radiation exposure evolve slowly over time, and symptoms may not manifest for days to weeks after exposure. Consider CRI in the differential diagnosis if the patient presents with a skin lesion without a history of chemical or thermal burn, insect bite, or skin disease or allergy. If the patient gives a history of possible radiation exposure (such as from a radiography source, x-ray device, or accelerator) or a history of finding and handling an unknown metallic object, note the presence of any of the following: erythema, blistering, dry or wet desquamation, epilation, ulceration.
Regarding lesions associated with CRI be aware that,
- days to weeks may pass before lesions appear;
- unless patients are symptomatic, they will not require emergency care; and
- lesions can be debilitating and life threatening after several weeks.
Medical follow-up is essential, and victims should be cautioned to avoid trauma to the involved areas.
Initial Treatment
Localized injuries should be treated symptomatically as they occur, and radiation injury experts should be consulted for detailed information. Such information can be obtained from the Radiation Emergency Assistance Center/Training Site (REAC/TS) at www.orau.gov/reacts/ or (865) 576-1005.
As with ARS, if the patient also has other trauma, wounds should be closed, burns covered, fractures reduced, surgical stabilization performed, and definitive treatment given within the first 48 hours after injury. After 48 hours, surgical interventions should be delayed until hematopoietic recovery has occurred.
A baseline CBC and differential should be taken and repeated in 24 hours. Because cutaneous radiation injury is cyclic, areas of early erythema should be noted and recorded. These areas should also be sketched and photographed, if possible, ensuring that the date and time are recorded. The following should be initiated as indicated:
- Supportive care in a clean environment (a burn unit if one is available)
- Prevention and treatment of infections
- Use of the following:
- Medications to reduce inflammation, inhibit protealysis, relieve pain, stimulate regeneration, and improve circulation
- Anticoagulant agents for widespread and deep injury
- Pain management
- Psychological support
Recommendations for Treatment by Stage
The following recommendations for treatment by stage of the illness were obtained by summarizing recommendations from Ricks et al. (226) and Gusev et al. (231), but they do not represent official recommendations of CDC.
- Prodromal Stage —Use antihistamines and topical antipruriginous preparations, which act against itch and also might prevent or attenuate initiation of the cycle that leads to the manifestation stage. Anti-inflammatory medications such as corticosteroids and topical creams, as well as slight sedatives, may prove useful.
- Latent Stage —Continue anti-inflammatory medications and sedatives. At midstage, use proteolysis inhibitors, such as Gordox®.
- Manifestation Stage —Use repeated swabs, antibiotic prophylaxis, and anti-inflammatory medications, such as Lioxasol®, to reduce bacterial, fungal, and viral infections
- Apply topical ointments containing corticosteroids along with locally acting antibiotics and vitamins.
- Stimulate regeneration of DNA by using Lioxasol® and later, when regeneration has started, biogenic drugs, such as Actovegin® and Solcoseril®.
- Stimulate blood supply in third or fourth week using Pentoxifylline® (contraindicated for patients with atherosclerotic heart disease).
- Puncture blisters if they are sterile, but do not remove them as long as they are intact.
- Stay alert for wound infection. Antibiotic therapy should be considered according to the individual patient's condition.
- Treat pain according to the individual patient's condition. Pain relief is very difficult and is the most demanding part of the therapeutic process.
- Debride areas of necrosis thoroughly but cautiously.
Treatment of Late Effects
After immediate treatment of radiation injury, an often long and painful process of healing will ensue. The most important concerns are the following:
- Pain management
- Fibrosis or late ulcers
Note : Use of medication to stimulate vascularization, inhibit infection, and reduce fibrosis may be effective. Examples include Pentoxifylline®, vitamin E, and interferon gamma. Otherwise, surgery may be required.
- Necrosis
- Plastic/reconstructive surgery
Note : Surgical treatment is common. It is most effective if performed early in the treatment process. Full-thickness graft and microsurgery techniques usually provide the best results.
- Psychological effects, such as posttraumatic stress disorder
- Possibility of increased risk of skin cancer later in life
Appendix A: Responses of the Skin to Radiation
- Acute epidermal necrosis (time of onset: < 10 days postexposure; threshold dose: ~550 Gy or 55,000 rads)— Interphase death of postmitotic keratinocytes in the upper visible layers of the epidermis (may occur with high-dose, low-energy beta irradiation)
- Acute ulceration (time of onset: < 14 days postexposure; threshold dose: ~20 Gy or 2000 rads)—Early loss of the epidermis— and to a varying degree, deeper dermal tissue—that results from the death of fibroblasts and endothelial cells in interphase
- Dermal atrophy (time of onset: > 26 weeks postexposure; threshold dose: ~10 Gy or 1000 rads)— Thinning of the dermal tissues associated with the contraction of the previously irradiated area
- Dermal necrosis (time of onset > 10 weeks postexposure; threshold dose: ~20 Gy or 2000 rads)— Necrosis of the dermal tissues as a consequence of vascular insufficiency
- Dry desquamation (time of onset: 3–6 weeks postexposure; threshold dose: ~8 Gy or 800 rads)— Atypical keratinization of the skin caused by the reduction in the number of clonogenic cells within the basal layer of the epidermis
- Early transient erythema (time of onset: within hours of exposure; threshold dose: ~2 Gray [Gy] or 200 rads)— Inflammation of the skin caused by activation of a proteolytic enzyme that increases the permeability of the capillaries
- Epilation (time of onset: 14–21 days; threshold dose: ~3 Gy or 300 rads)— Hair loss caused by the depletion of matrix cells in the hair follicles
- Late erythema (time of onset: 8–20 weeks postexposure; threshold dose: ~20 Gy or 2000 rads)— Inflammation of the skin caused by injury of blood vessels. Edema and impaired lymphatic clearance precede a measured reduction in blood flow.
- Invasive fibrosis (time of onset: months to years postexposure; threshold dose: ~20 Gy or 2000 rads)— Method of healing associated with acute ulceration, secondary ulceration, and dermal necrosis that leads to scar tissue formation
- Main erythema (time of onset: days to weeks postexposure; threshold dose: ~3 Gy or 300 rads)— Inflammation of the skin caused by hyperaemia of the basal cells and subsequent epidermal hypoplasia (see photos 1 and 2)
- Moist desquamation (time of onset: 4–6 weeks postexposure; threshold dose: ~15 Gy or 1500 rads)— Loss of the epidermis caused by sterilization of a high proportion of clonogenic cells within the basal layer of the epidermis
- Secondary ulceration (time of onset: > 6 weeks postexposure; threshold dose: ~15 Gy or 1500 rads)— Secondary damage to the dermis as a consequence of dehydration and infection when moist desquamation is severe and protracted because of reproductive sterilization of the vast majority of the clonogenic cells in the irradiated area
- Telangiectasia (time of onset: > 52 weeks postexposure; threshold dose for moderate severity at 5 years: ~40 Gy or 4000 rads)— Atypical dilation of the superficial dermal capillaries.
Appendix B: Images
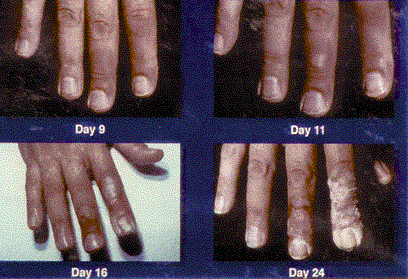
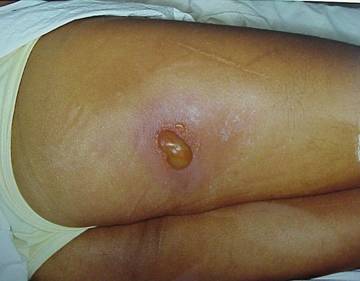
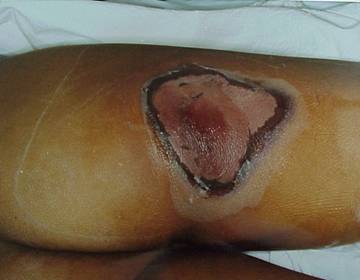
Figures 1 & 2 . Erythema: These photos display the progression of erythema in a patient involved in an x-ray diffraction accident, 9 days to 96 days postexposure. The day following the exposure (not shown), the patient displayed only mild diffuse swelling and erythema of the fingertips. On day 9, punctuate lesions resembling telangiectasias were noted in the subungal region of the right index finger, and on day 11, blisters began to appear. Desquamation continued for several weeks. The patient developed cellulitis in the right thumb approximately 2 years following exposure. The area of the right fingertip and nail continued to cause the patient great pain when even minor trauma occurred to the fingertip, and he required occasional oral narcotic analgesics to manage this pain. He continued to experience intense pain resulting from minor trauma to the affected areas for as long as 4 years postexposure.
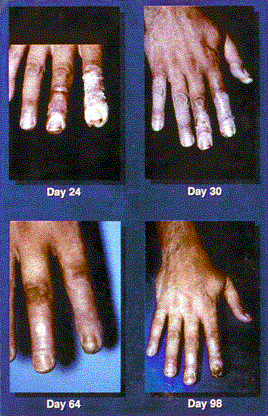
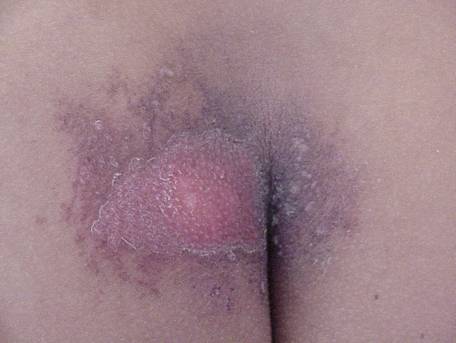
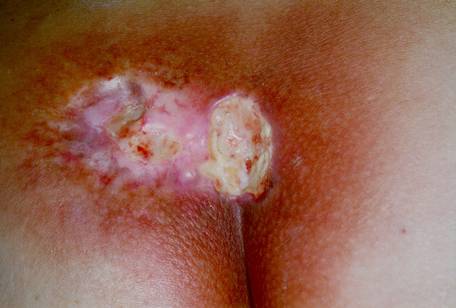
Figures 3 & 4. Acute ulceration. These photos show acute ulceration in a Peruvian patient who inadvertently placed a 26-Ci (0.962-TBq) irridiun-192 ( 192 Ir) source in his back pocket, 3 days and 10 days postexposure. The source remained in the patient's pocket for approximately 6.5 hours, at which time he complained to his wife about pain in his posterior right thigh. He sought medical advice and was told he probably had been bitten by an insect. In the meantime, his wife sat on the patient's pants (her case appears on the next page) while breastfeeding the couple's 1½-year-old child. The source was recovered several hours later by nuclear regulatory authorities, and the patient was transported to Lima for treatment. This patient exhibited a drastic reduction in lymphocyte count by day 3 postexposure, and a 4-by-4-cm lesion appeared on day 4. Eventually he suffered with a massive ulceration and necrosis of the site with infection, and his right leg was amputated. Grade II and III CRI was also evident on his hands, left leg, and perineum, but he survived and returned to his family.
Acute Radiation Syndrome: A Fact Sheet for Physicians
Acute Radiation Syndrome (ARS) (sometimes known as radiation toxicity or radiation sickness) is an acute illness caused by irradiation of the entire body (or most of the body) by a high dose of penetrating radiation in a very short period of time (usually a matter of minutes). The major cause of this syndrome is depletion of immature parenchymal stem cells in specific tissues. Examples of people who suffered from ARS are the survivors of the Hiroshima and Nagasaki atomic bombs, the firefighters that first responded after the Chernobyl Nuclear Power Plant event in 1986, and some unintentional exposures to sterilization irradiators.
The required conditions for Acute Radiation Syndrome (ARS) are:
- The radiation dose must be large (i.e., greater than 0.7 Gray (Gy)1, 2 or 70 rads).
- Mild symptoms may be observed with doses as low as 0.3 Gy or 30 rads.
- The dose usually must be external ( i.e., the source of radiation is outside of the patient’s body).
- Radioactive materials deposited inside the body have produced some ARS effects only in extremely rare cases.
- The radiation must be penetrating (i.e., able to reach the internal organs).
- High energy X-rays, gamma rays, and neutrons are penetrating radiations.
- The entire body (or a significant portion of it) must have received the dose3.
- Most radiation injuries are local, frequently involving the hands, and these local injuries seldom cause classical signs of ARS.
- The dose must have been delivered in a short time (usually a matter of minutes).
- Fractionated doses are often used in radiation therapy. These are large total doses delivered in small daily amounts over a period of time. Fractionated doses are less effective at inducing ARS than a single dose of the same magnitude.
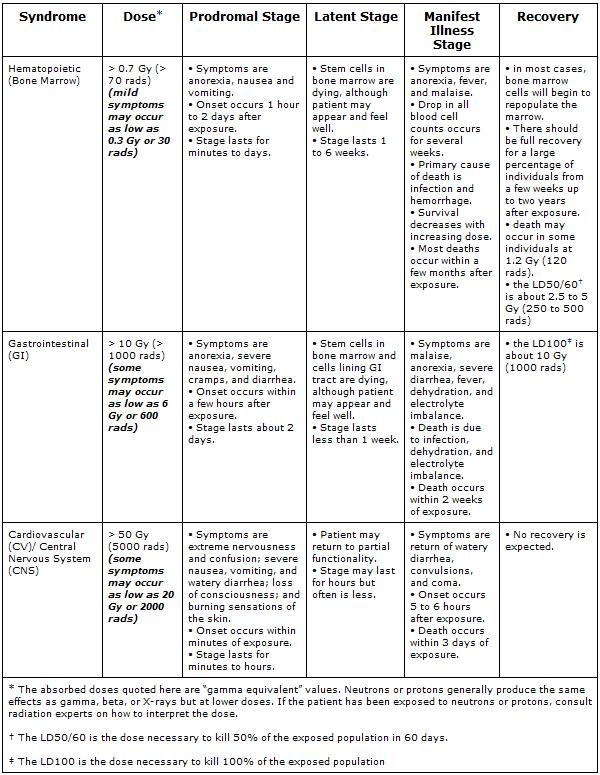
The three classic Acute Radiation Syndromes are
- Bone marrow syndrome (sometimes referred to as hematopoietic syndrome) the full syndrome will usually occur with a dose between 0.7 and 10 Gy (70 – 1000 rads) though mild symptoms may occur as low as 0.3 Gy or 30 rads4.
- The survival rate of patients with this syndrome decreases with increasing dose. The primary cause of death is the destruction of the bone marrow, resulting in infection and hemorrhage.
- Gastrointestinal (GI) syndrome: the full syndrome will usually occur with a dose greater than approximately 10 Gy (1000 rads) although some symptoms may occur as low as 6 Gy or 600 rads.
- Survival is extremely unlikely with this syndrome. Destructive and irreparable changes in the GI tract and bone marrow usually cause infection, dehydration, and electrolyte imbalance. Death usually occurs within 2 weeks.
- Cardiovascular (CV)/ Central Nervous System (CNS) syndrome: the full syndrome will usually occur with a dose greater than approximately 50 Gy (5000 rads) although some symptoms may occur as low as 20 Gy or 2000 rads.
- Death occurs within 3 days. Death likely is due to collapse of the circulatory system as well as increased pressure in the confining cranial vault as the result of increased fluid content caused by edema, vasculitis, and meningitis.
The four stages of ARS are
- Prodromal stage (N-V-D stage): The classic symptoms for this stage are nausea, vomiting, as well as anorexia and possibly diarrhea (depending on dose), which occur from minutes to days following exposure. The symptoms may last (episodically) for minutes up to several days.
- Latent stage: In this stage, the patient looks and feels generally healthy for a few hours or even up to a few weeks.
- Manifest illness stage: In this stage the symptoms depend on the specific syndrome (see Table 1) and last from hours up to several months.
- Recovery or death: Most patients who do not recover will die within several months of exposure. The recovery process lasts from several weeks up to two years.
Cutaneous Radiation Syndrome (CRS)
The concept of cutaneous radiation syndrome (CRS) was introduced in recent years to describe the complex pathological syndrome that results from acute radiation exposure to the skin.
ARS usually will be accompanied by some skin damage. It is also possible to receive a damaging dose to the skin without symptoms of ARS, especially with acute exposures to beta radiation or X-rays. Sometimes this occurs when radioactive materials contaminate a patient’s skin or clothes.
When the basal cell layer of the skin is damaged by radiation, inflammation, erythema, and dry or moist desquamation can occur. Also, hair follicles may be damaged, causing epilation. Within a few hours after irradiation, a transient and inconsistent erythema (associated with itching) can occur. Then, a latent phase may occur and last from a few days up to several weeks, when intense reddening, blistering, and ulceration of the irradiated site are visible. In most cases, healing occurs by regenerative means; however, very large skin doses can cause permanent hair loss, damaged sebaceous and sweat glands, atrophy, fibrosis, decreased or increased skin pigmentation, and ulceration or necrosis of the exposed tissue. Patient Management
Triage: If radiation exposure is suspected:
- Secure ABCs (airway, breathing, circulation) and physiologic monitoring (blood pressure, blood gases, electrolyte and urine output) as appropriate.
- Treat major trauma, burns and respiratory injury if evident.
- In addition to the blood samples required to address the trauma, obtain blood samples for CBC (complete blood count), with attention to lymphocyte count, and HLA (human leukocyte antigen) typing prior to any initial transfusion and at periodic intervals following transfusion.
- Treat contamination as needed.
- If exposure occurred within 8 to 12 hours, repeat CBC, with attention to lymphocyte count, 2 or 3 more times (approximately every 2 to 3 hours) to assess lymphocyte depletion.
Diagnosis
The diagnosis of ARS can be difficult to make because ARS causes no unique disease. Also, depending on the dose, the prodromal stage may not occur for hours or days after exposure, or the patient may already be in the latent stage by the time they receive treatment, in which case the patient may appear and feel well when first assessed.
If a patient received more than 0.05 Gy (5 rads) and three or four CBCs are taken within 8 to 12 hours of the exposure, a quick estimate of the dose can be made (see Ricks, et. al. for details). If these initial blood counts are not taken, the dose can still be estimated by using CBC results over the first few days. It would be best to have radiation dosimetrists conduct the dose assessment, if possible.
If a patient is known to have been or suspected of having been exposed to a large radiation dose, draw blood for CBC analysis with special attention to the lymphocyte count, every 2 to 3 hours during the first 8 hours after exposure (and every 4 to 6 hours for the next 2 days). Observe the patient during this time for symptoms and consult with radiation experts before ruling out ARS.
If no radiation exposure is initially suspected, you may consider ARS in the differential diagnosis if a history exists of nausea and vomiting that is unexplained by other causes. Other indications are bleeding, epilation, or white blood count (WBC) and platelet counts abnormally low a few days or weeks after unexplained nausea and vomiting. Again, consider CBC and chromosome analysis and consultation with radiation experts to confirm diagnosis.
Initial Treatment and Diagnostic Evaluation
Treat vomiting, and repeat CBC analysis, with special attention to the lymphocyte count, every 2 to 3 hours for the first 8 to 12 hours following exposure (and every 4 to 6 hours for the following 2 or 3 days). Sequential changes in absolute lymphocyte counts over time are demonstrated below in the Andrews Lymphocyte Nomogram (see Figure 1). Precisely record all clinical symptoms, particularly nausea, vomiting, diarrhea, and itching, reddening or blistering of the skin. Be sure to include time of onset.
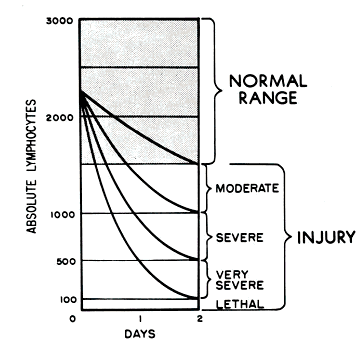
Note and record areas of erythema. If possible, take color photographs of suspected radiation skin damage. Consider tissue, blood typing, and initiating viral prophylaxis. Promptly consult with radiation, hematology, and radiotherapy experts about dosimetry, prognosis, and treatment options. Call the Radiation Emergency Assistance Center/Training Site (REAC/TS) at (865) 576-3131 (M-F, 8 am to 4:30 am EST) or (865) 576-1005 (after hours) to record the incident in the Radiation Accident Registry System.
After consultation, begin the following (as indicated):
- supportive care in a clean environment (if available, the use of a burn unit may be quite effective)
- prevention and treatment of infections
- stimulation of hematopoiesis by use of growth factors
- stem cell transfusions or platelet transfusions (if platelet count is too low)
- psychological support
- careful observation for erythema (document locations), hair loss, skin injury, mucositis, parotitis, weight loss, or fever
- confirmation of initial dose estimate using chromosome aberration cytogenetic bioassay when possible. Although resource intensive, this is the best method of dose assessment following acute exposures.
- consultation with experts in radiation accident management.
Diagnostic Findings
Plain X-ray
-
Pelvic radiograph demonstrates radiation osteonecrosis
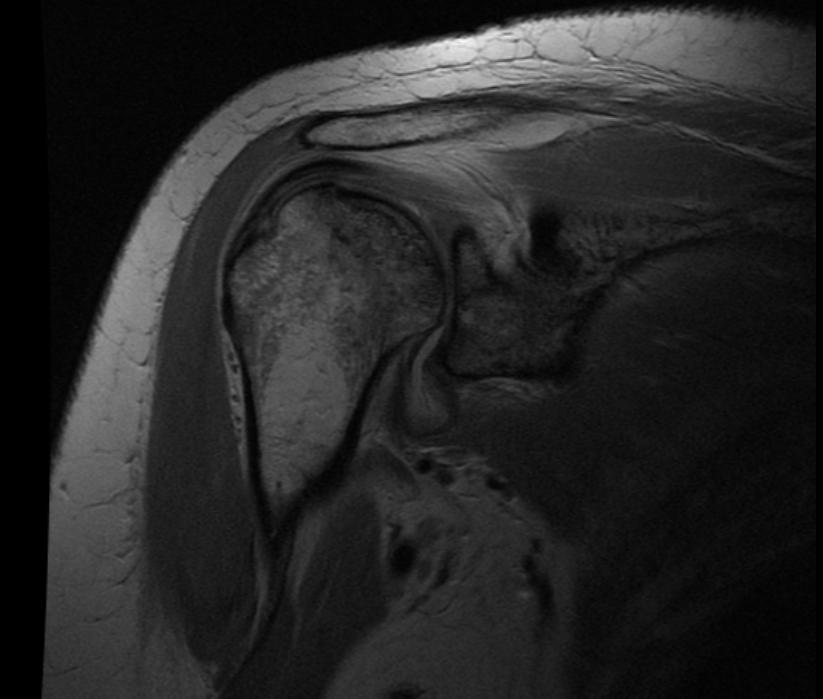
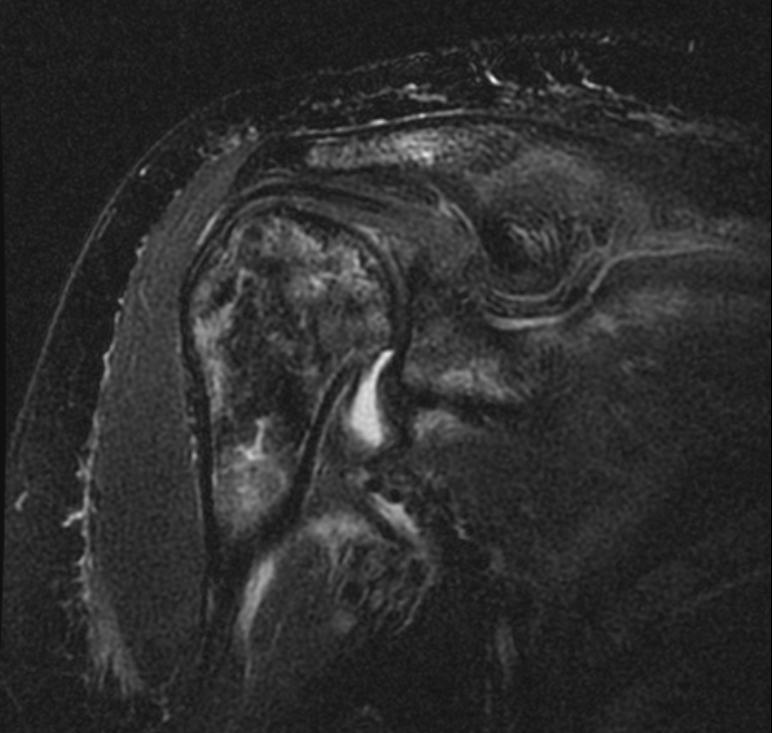
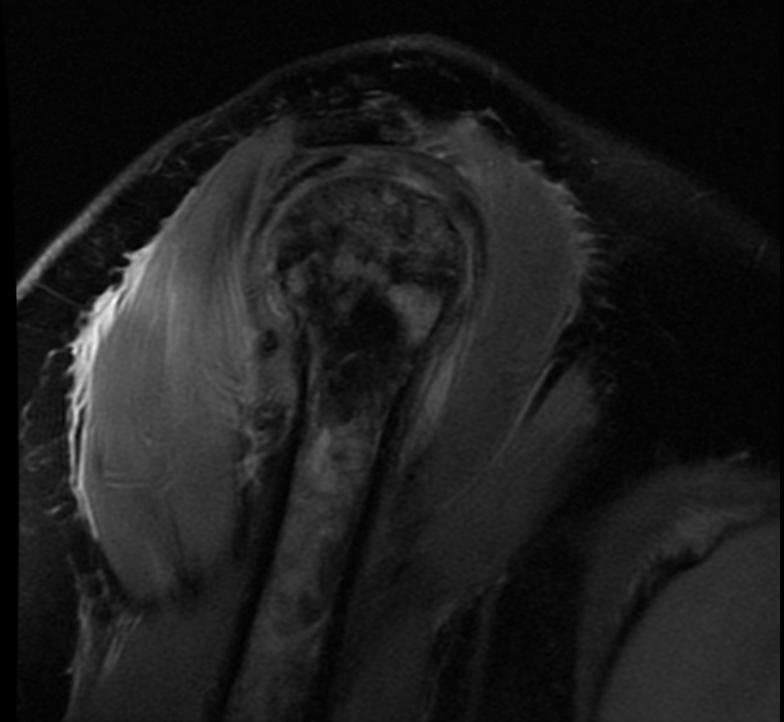
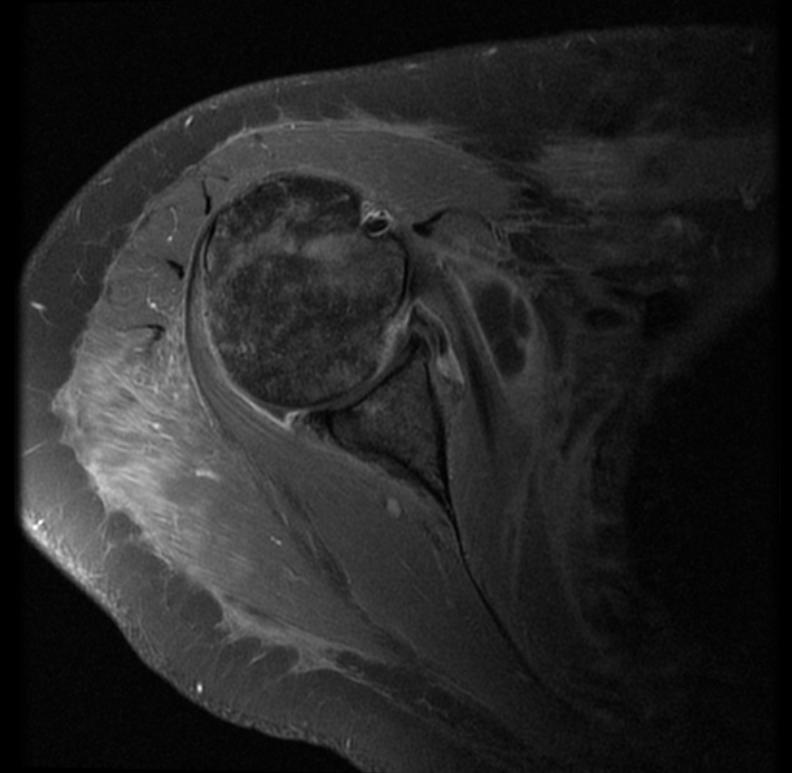
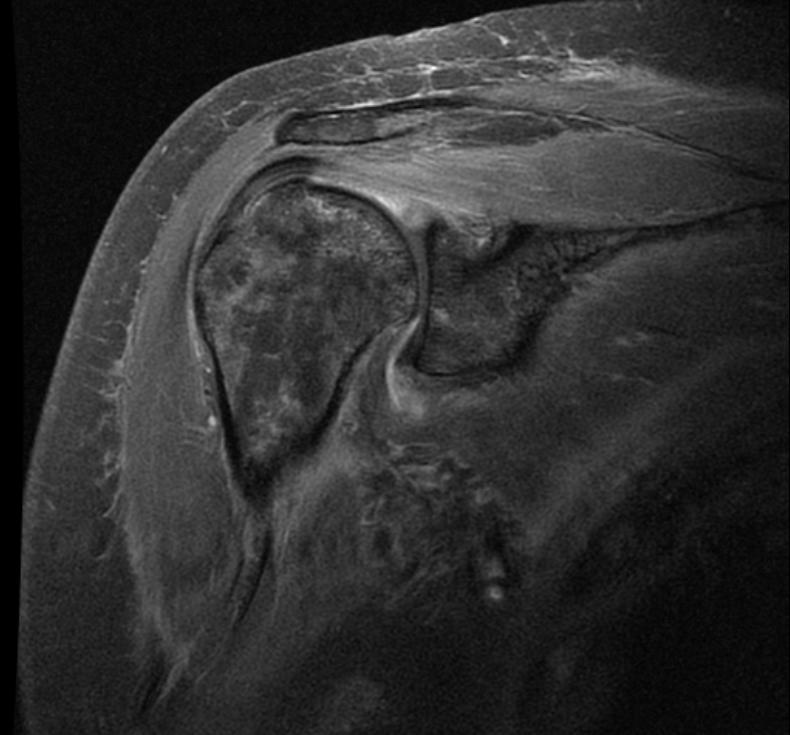
MRI
-
Radiation necrosis
-
Radiation necrosis
-
Radiation necrosis
-
Radiation necrosis
-
Radiation necrosis
Case Examples
Case #1
Clinical Summary
This 60-year-old white female had developed retraction of her left nipple six years earlier, at which time breast carcinoma was found. A radical mastectomy was performed. Examination of the surgical specimens showed metastases in regional lymph nodes and local irradiation was thus administered.
Two years later, carcinoma of the right breast was found. Following a modified mastectomy, more irradiation was given. A year later the patient developed recurrences for which chemotherapy (cytoxan and adriamycin) was given. After a two year period without problems, the patient developed decreased exercise tolerance, dyspnea on exertion, shortness of breath, paroxysmal nocturnal dyspnea, and orthopnea increasing in severity over 10 days. Chest examination revealed decreased breath sounds with dullness over the left base. Chest x-ray showed a globose cardiac silhouette and left pleural effusion.
A pericardiectomy was done because of suspected cardiac tamponade; however, the patient died soon after the operation.
Autopsy Findings
There was metastatic carcinoma in the pericardium, chest wall, diaphragm, both lungs, and mediastinal lymph nodes. Severe nonobstructive cardiomyopathy, probably secondary to adriamycin, was found. Areas of pleural thickening with adhesions and interstitial fibrosis were found involving the anterior aspect of both lungs.
Histopathological Findings
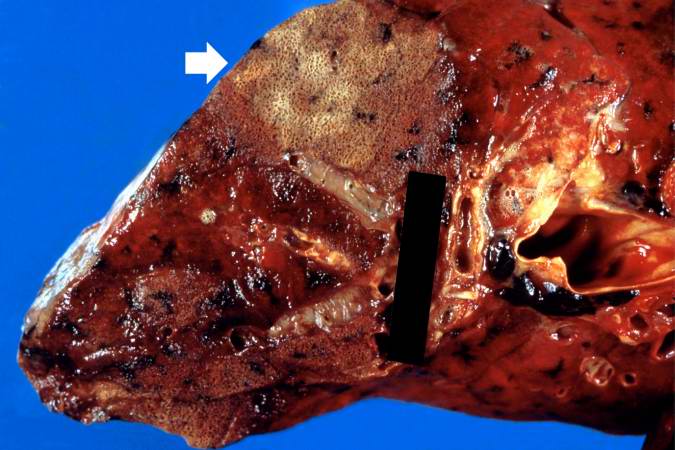
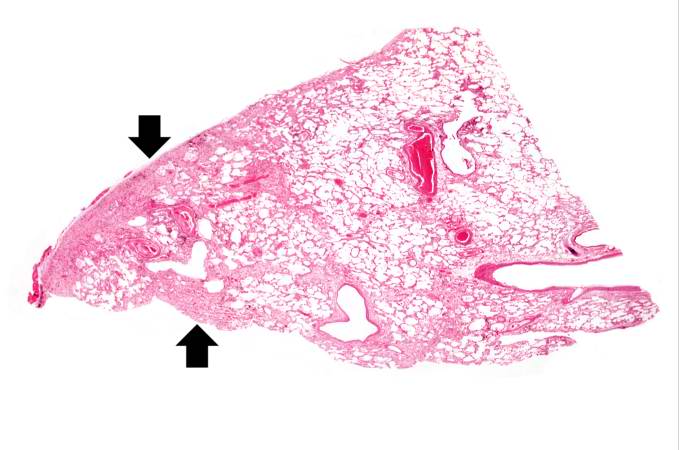
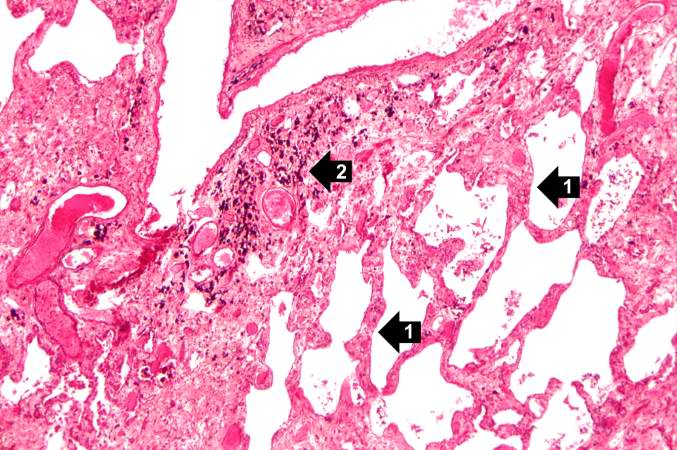
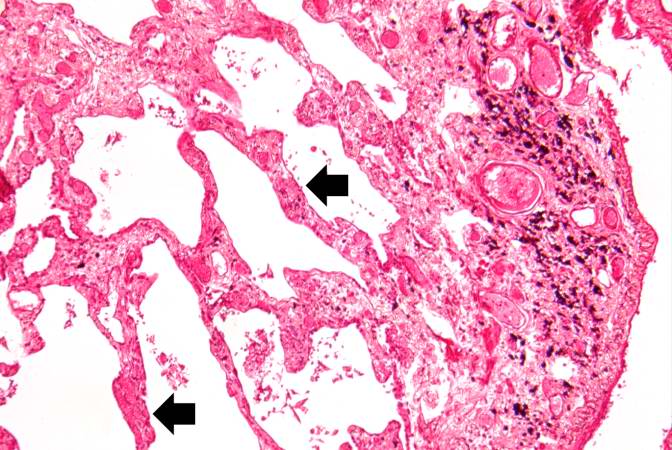
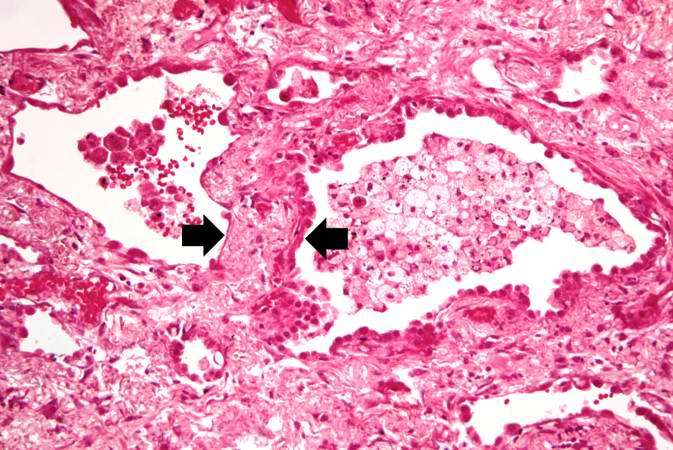
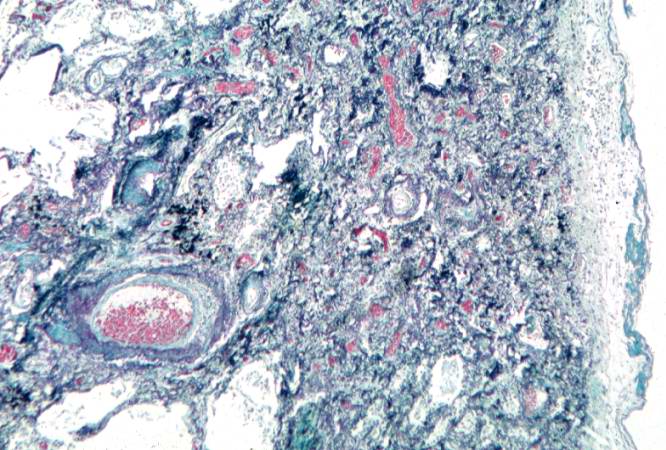
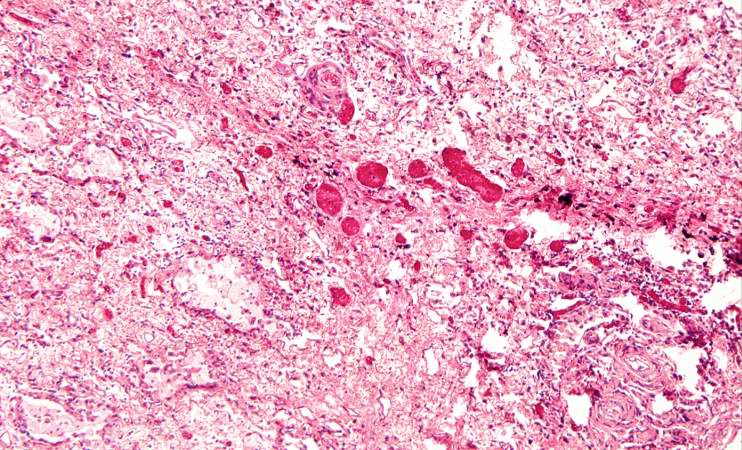
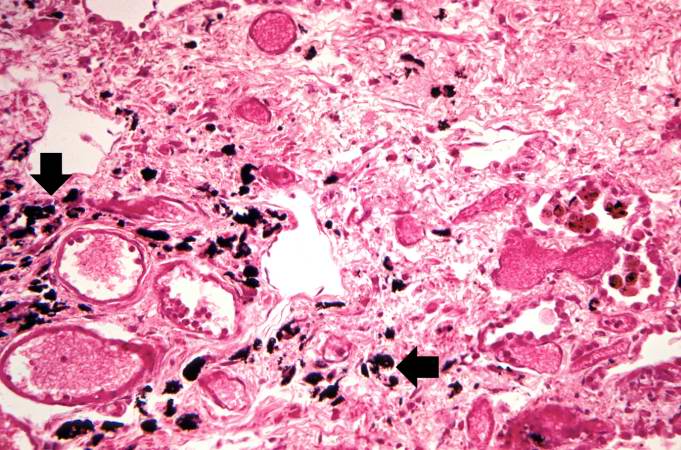
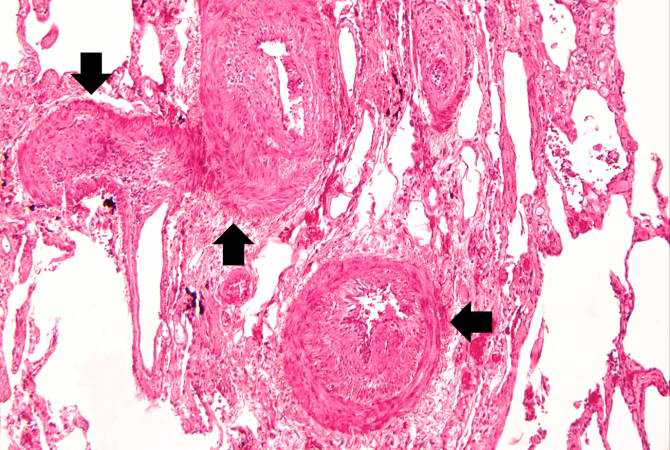
Source
References
- Berger ME, O’Hare FM Jr, Ricks RC, editors. The Medical Basis for Radiation Accident Preparedness: The Clinical Care of Victims. REAC/TS Conference on the Medical Basis for Radiation Accident Preparedness. New York : Parthenon Publishing; 2002.
- Gusev IA , Guskova AK , Mettler FA Jr, editors. Medical Management of Radiation Accidents, 2 nd ed., New York : CRC Press, Inc.; 2001.
- Jarrett DG. Medical Management of Radiological Casualties Handbook, 1 st ed. Bethesda , Maryland : Armed Forces Radiobiology Research Institute (AFRRI); 1999.
- LaTorre TE. Primer of Medical Radiobiology, 2 nd ed. Chicago : Year Book Medical Publishers, Inc.; 1989.
- National Council on Radiation Protection and Measurements (NCRP). Management of Terrorist Events Involving Radioactive Material, NCRP Report No. 138. Bethesda , Maryland : NCRP; 2001.
- Prasad KN. Handbook of Radiobiology, 2 nd ed. New York : CRC Press, Inc.; 1995.
Additional Resources
- Michihiko Hachiya, Hiroshima Diary (Chapel Hill: University of North Carolina, 1955), ISBN 0-8078-4547-7.
- John Hersey, Hiroshima (New York: Vintage, 1946, 1985 new chapter), ISBN 0-679-72103-7.
- Ibuse Masuji, Black Rain (1969) ISBN 0-87011-364-X
- Ernest J. Sternglass, Secret Fallout: low-level radiation from Hiroshima to Three-Mile Island (1981) ISBN 0-07-061242-0 (online)
- Norman Solomon, Harvey Wasserman Killing Our Own: The Disaster of America's Experience with Atomic Radiation, 1945-1982, New York: Dell, 1982. ISBN 0-385-28537-X, ISBN 0-385-28536-1, ISBN 0-440-04567-3 (online)
External links
- Radiation accidents with multi-organ failure in the United States
- List of radiation accidents and other events causing radiation casualties
- The critical accident in Sarov, International Atomic Energy Agency
- The Center for Disease Control's fact sheet on Acute Radiation Syndrome
- Therac-25 computerized radiation therapy machine accidents
Template:Consequences of external causes
Template:SIB
- ↑ Wynn A. Volkert and Timothy J. Hoffman, Therapeutic Radiopharmaceuticals, Chemical Reviews 99(9) (1999); 2269–2292