Pulmonary alveolar proteinosis: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{SI}} | {{SI}} | ||
{{CMG}} {{AE}}{{MA}} [mailto:malihash@bidmc.harvard.edu] [mailto:malihash@bidmc.harvard.edu] [mailto:malihash@bidmc.harvard.edu] [mailto:malihash@bidmc.harvard.edu] [mailto:malihash@bidmc.harvard.edu] [mailto:malihash@bidmc.harvard.edu] | {{CMG}} {{AE}}{{MA}} [mailto:malihash@bidmc.harvard.edu] [mailto:malihash@bidmc.harvard.edu] [mailto:malihash@bidmc.harvard.edu] [mailto:malihash@bidmc.harvard.edu] [mailto:malihash@bidmc.harvard.edu] [mailto:malihash@bidmc.harvard.edu] [mailto:malihash@bidmc.harvard.edu] | ||
{{SK}}Pulmonary alveolar phospholipoproteinosis ; Synonym 2; Synonym 3 | {{SK}}Pulmonary alveolar phospholipoproteinosis ; Synonym 2; Synonym 3 | ||
| Line 25: | Line 25: | ||
:** Variants of ATP-binding cassette, subfamily A (ABCA3)<ref name="pmid11940594">{{cite journal |vauthors=Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H |title=Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3 |journal=J. Biol. Chem. |volume=277 |issue=25 |pages=22147–55 |date=June 2002 |pmid=11940594 |doi=10.1074/jbc.M201812200 |url=}}</ref> | :** Variants of ATP-binding cassette, subfamily A (ABCA3)<ref name="pmid11940594">{{cite journal |vauthors=Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H |title=Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3 |journal=J. Biol. Chem. |volume=277 |issue=25 |pages=22147–55 |date=June 2002 |pmid=11940594 |doi=10.1074/jbc.M201812200 |url=}}</ref> | ||
:** Variant of NK2 homeobox-1 (NKX2.1) <ref name="pmid23997037">{{cite journal |vauthors=Salerno T, Peca D, Menchini L, Schiavino A, Petreschi F, Occasi F, Cogo P, Danhaive O, Cutrera R |title=Respiratory insufficiency in a newborn with congenital hypothyroidism due to a new mutation of TTF-1/NKX2.1 gene |journal=Pediatr. Pulmonol. |volume=49 |issue=3 |pages=E42–4 |date=March 2014 |pmid=23997037 |doi=10.1002/ppul.22788 |url=}}</ref> | :** Variant of NK2 homeobox-1 (NKX2.1) <ref name="pmid23997037">{{cite journal |vauthors=Salerno T, Peca D, Menchini L, Schiavino A, Petreschi F, Occasi F, Cogo P, Danhaive O, Cutrera R |title=Respiratory insufficiency in a newborn with congenital hypothyroidism due to a new mutation of TTF-1/NKX2.1 gene |journal=Pediatr. Pulmonol. |volume=49 |issue=3 |pages=E42–4 |date=March 2014 |pmid=23997037 |doi=10.1002/ppul.22788 |url=}}</ref> | ||
:** Variants in the gene SLC7A7< | :** Variants in the gene SLC7A7<ref name="urlGeneReviews® - NCBI Bookshelf">{{cite web |url=https://www.ncbi.nlm.nih.gov/books/NBK1116/ |title=GeneReviews® - NCBI Bookshelf |format= |work= |accessdate=}}</ref><nowiki/> | ||
:** Variants of the MARS (Methionyl-tRNA synthetasegene ): Prevalent on Réunion Island<ref name="pmid25913036">{{cite journal |vauthors=Hadchouel A, Wieland T, Griese M, Baruffini E, Lorenz-Depiereux B, Enaud L, Graf E, Dubus JC, Halioui-Louhaichi S, Coulomb A, Delacourt C, Eckstein G, Zarbock R, Schwarzmayr T, Cartault F, Meitinger T, Lodi T, de Blic J, Strom TM |title=Biallelic Mutations of Methionyl-tRNA Synthetase Cause a Specific Type of Pulmonary Alveolar Proteinosis Prevalent on Réunion Island |journal=Am. J. Hum. Genet. |volume=96 |issue=5 |pages=826–31 |date=May 2015 |pmid=25913036 |pmc=4570277 |doi=10.1016/j.ajhg.2015.03.010 |url=}}</ref> | :** Variants of the MARS (Methionyl-tRNA synthetasegene ): Prevalent on Réunion Island<ref name="pmid25913036">{{cite journal |vauthors=Hadchouel A, Wieland T, Griese M, Baruffini E, Lorenz-Depiereux B, Enaud L, Graf E, Dubus JC, Halioui-Louhaichi S, Coulomb A, Delacourt C, Eckstein G, Zarbock R, Schwarzmayr T, Cartault F, Meitinger T, Lodi T, de Blic J, Strom TM |title=Biallelic Mutations of Methionyl-tRNA Synthetase Cause a Specific Type of Pulmonary Alveolar Proteinosis Prevalent on Réunion Island |journal=Am. J. Hum. Genet. |volume=96 |issue=5 |pages=826–31 |date=May 2015 |pmid=25913036 |pmc=4570277 |doi=10.1016/j.ajhg.2015.03.010 |url=}}</ref> | ||
Revision as of 16:11, 5 March 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Mahda Alihashemi M.D. [2] [3] [4] [5] [6] [7] [8] [9]
Synonyms and keywords:Pulmonary alveolar phospholipoproteinosis ; Synonym 2; Synonym 3
Overview
Historical Perspective
- Pulmonary alveolar proteinosis ( PAP) was first discovered by Samuel Rosen, Benjamin Castleman, and Averill Liebow, in 1958 during pathologic investigations of material filling the alveoli. [1]
- In [year], [gene] mutations were first identified in the pathogenesis of [disease name].
- In 1960, the first therapeutic bronchoalveolar lavage by repeated segmental flooding was developed by Dr. Jose Ramirez-Rivera to treat pulmonary alveolar proteinosis. [2]
Classification
Pulmonary alveolar proteinosis ( PAP)may be classified into 3 subtypes:
- Autoimmune and hereditary PAP: Disruption of Granulocyte-macrophage colony-stimulating factor (GM-CSF) signalling[3]
- Clearance of surfactant by alveolar macrophages is regulated by GM-CSF
- Autoimmune PAP: The most common type of PAP in adults
- Effect of GM-CSF on alveolar macrophages is neutralized by antibodies to GM-CSF
- Hereditary PAP :GM-CSF is intact but recessive variants of the GM-CSF receptor alpha and beta genes (CSF2RA and CSF2RB) impair signaling by GM-CSF
- Autoimmune and hereditary PAP: Disruption of Granulocyte-macrophage colony-stimulating factor (GM-CSF) signalling[3]
- Congenital PAP: Disorders of surfactant production
- Variants in surfactant proteins (SFTPB and SFTPC) [4-6] •Variants in proteins involved in the metabolism of surfactant (ATP-binding cassette, subfamily A [ABCA3]) [7] •Variants in NK2 homeobox-1 (NKX2.1) thyroid transcription factor-1 (TTF1), which regulates transcription of SFTPB, SFTPC, and ABCA3 [8] •Variants in the gene SLC7A7, which causes a defect in the plasma membrane transport of cationic amino acids (known as lysinuric protein intolerance). (See "Pulmonary alveolar proteinosis in children", section on 'Congenital form'.) Methionyl-tRNA synthetase (MARS) catalyzes the incorporation of methionine into tRNA and is critical for protein biosynthesis. Genetic variants of the MARS gene are associated with a rare childhood form of PAP prevalent on Réunion Island. (See "Genetic disorders of surfactant dysfunction", section on 'Related disorders'.)
- Variants of surfactant proteins B and C (SFTPB, SFTPC)
| Variants of surfactant proteins B and C (SFTPB, SFTPC) |
| Variants of ATP-binding cassette, subfamily A (ABCA3) |
| Variant of NK2 homeobox-1 (NKX2.1) that encodes thyroid transcription factor-1 (TTF1), which in turn regulates transcription of SFTP-C and ABCA3 |
| Variants of SLC7A7/y+LAT1, which causes lysinuric protein intolerance (LPI). Patients with LPI have defective cationic amino acid transport (y+L transport) and defective expression of y+LAT in alveolar macrophages |
| Methionyl-tRNA synthetase (MARS) variants: Compound heterozygosity associated with liver failure, PAP, anemia, delays in motor development; exact mechanism of PAP uncertain |
| Variants of surfactant proteins B and C (SFTPB, SFTPC) |
| Variants of ATP-binding cassette, subfamily A (ABCA3) |
| Variant of NK2 homeobox-1 (NKX2.1) that encodes thyroid transcription factor-1 (TTF1), which in turn regulates transcription of SFTP-C and ABCA3 |
| Variants of SLC7A7/y+LAT1, which causes lysinuric protein intolerance (LPI). Patients with LPI have defective cationic amino acid transport (y+L transport) and defective expression of y+LAT in alveolar macrophages |
| Methionyl-tRNA synthetase (MARS) variants: Compound heterozygosity associated with liver failure, PAP, anemia, delays in motor development; exact mechanism of PAP uncertain |
- Other variants of [disease name] include [disease subtype 1], [disease subtype 2], and [disease subtype 3].
DEFINITIONS AND CLASSIFICATION — PAP is caused by a spectrum of disorders that negatively affect production and clearance of surfactant. Three main categories of PAP are recognized (table 1) [2,3]:
●Disruption of granulocyte-macrophage colony-stimulating factor signaling (autoimmune and hereditary PAP) – Granulocyte-macrophage colony-stimulating factor (GM-CSF) regulates clearance of surfactant by alveolar macrophages. Disorders that disrupt GM-CSF signalling include autoimmune PAP (the most common type of PAP in adults) and hereditary PAP due to recessive variants of the GM-CSF receptor alpha and beta genes (CSF2RA and CSF2RB). Antibodies to GM-CSF neutralize the effect of GM-CSF on alveolar macrophages, while genetic variants in the GM-CSF receptor impair signaling by intact GM-CSF.
●Disorders of surfactant production (congenital PAP) – Disorders of surfactant production are traditionally considered congenital PAP. These disorders often present in the neonatal period and encompass a number of genetic variants:
•Variants in surfactant proteins (SFTPB and SFTPC) [4-6]
•Variants in proteins involved in the metabolism of surfactant (ATP-binding cassette, subfamily A [ABCA3]) [7]
•Variants in NK2 homeobox-1 (NKX2.1) thyroid transcription factor-1 (TTF1), which regulates transcription of SFTPB, SFTPC, and ABCA3 [8]
•Variants in the gene SLC7A7, which causes a defect in the plasma membrane transport of cationic amino acids (known as lysinuric protein intolerance). (See "Pulmonary alveolar proteinosis in children", section on 'Congenital form'.)
Methionyl-tRNA synthetase (MARS) catalyzes the incorporation of methionine into tRNA and is critical for protein biosynthesis. Genetic variants of the MARS gene are associated with a rare childhood form of PAP prevalent on Réunion Island. (See "Genetic disorders of surfactant dysfunction", section on 'Related disorders'.)
Congenital alveolar proteinosis is discussed in greater detail separately. (See "Pulmonary alveolar proteinosis in children".)
●Secondary PAP – The secondary form of PAP develops in adulthood and is found in association with high level dust exposures (eg, silica, aluminum, titanium, indium-tin oxide) [9-18], hematologic dyscrasias (eg, myelodysplastic syndrome, GATA2 deficiency, hematologic malignancy) [19-24], and after allogeneic hematopoietic cell transplantation for myeloid malignancies [25-27]. (See "Pulmonary complications after allogeneic hematopoietic cell transplantation".)
In most cases, secondary PAP appears related to a relative deficiency of GM-CSF and related macrophage dysfunction [9-13,18-20]. High level dust inhalation appears to impair macrophage function through direct toxicity. Although indium-tin oxide inhalation has been associated with secondary PAP, in one report, one of two patients with indium-tin oxide-related PAP had elevated levels of antibodies to GM-CSF [14], raising the hypothesis that inhalation of an agent that causes PAP on lung pathology may be the consequence of an autoimmune process [28-30]. However, a subsequent study of 87 indium-tin oxide facility workers did not identify any workers with evidence of PAP or antibodies to GM-CSF [31]. Thus, it appears unlikely that PAP in this setting is an autoimmune process.
A few patients with cytologic or histopathologic evidence of PAP have reduced GM-CSF signalling, but no evidence of anti-GM-CSF antibodies or mutations in the GM-CSF receptor alpha or beta chains. These patients are considered to have unclassified PAP [2].
Pathophysiology
- The pathogenesis of [disease name] is characterized by [feature1], [feature2], and [feature3].
- The [gene name] gene/Mutation in [gene name] has been associated with the development of [disease name], involving the [molecular pathway] pathway.
- On gross pathology, [feature1], [feature2], and [feature3] are characteristic findings of [disease name].
- On microscopic histopathological analysis, [feature1], [feature2], and [feature3] are characteristic findings of [disease name].
Causes
- [Disease name] may be caused by either [cause1], [cause2], or [cause3].
- [Disease name] is caused by a mutation in the [gene1], [gene2], or [gene3] gene[s].
- There are no established causes for [disease name].
Differentiating [disease name] from other Diseases
- [Disease name] must be differentiated from other diseases that cause [clinical feature 1], [clinical feature 2], and [clinical feature 3], such as:
- [Differential dx1]
- [Differential dx2]
- [Differential dx3]
Epidemiology and Demographics
- The prevalence of [disease name] is approximately [number or range] per 100,000 individuals worldwide.
- In [year], the incidence of [disease name] was estimated to be [number or range] cases per 100,000 individuals in [location].
Age
- Patients of all age groups may develop [disease name].
- [Disease name] is more commonly observed among patients aged [age range] years old.
- [Disease name] is more commonly observed among [elderly patients/young patients/children].
Gender
- [Disease name] affects men and women equally.
- [Gender 1] are more commonly affected with [disease name] than [gender 2].
- The [gender 1] to [Gender 2] ratio is approximately [number > 1] to 1.
Race
- There is no racial predilection for [disease name].
- [Disease name] usually affects individuals of the [race 1] race.
- [Race 2] individuals are less likely to develop [disease name].
Risk Factors
- Common risk factors in the development of [disease name] are [risk factor 1], [risk factor 2], [risk factor 3], and [risk factor 4].
Natural History, Complications and Prognosis
- The majority of patients with [disease name] remain asymptomatic for [duration/years].
- Early clinical features include [manifestation 1], [manifestation 2], and [manifestation 3].
- If left untreated, [#%] of patients with [disease name] may progress to develop [manifestation 1], [manifestation 2], and [manifestation 3].
- Common complications of [disease name] include [complication 1], [complication 2], and [complication 3].
- Prognosis is generally [excellent/good/poor], and the [1/5/10year mortality/survival rate] of patients with [disease name] is approximately [#%].
Diagnosis
Diagnostic Criteria
- The diagnosis of [disease name] is made when at least [number] of the following [number] diagnostic criteria are met:
- [criterion 1]
- [criterion 2]
- [criterion 3]
- [criterion 4]
Symptoms
- [Disease name] is usually asymptomatic.
- Symptoms of [disease name] may include the following:
- [symptom 1]
- [symptom 2]
- [symptom 3]
- [symptom 4]
- [symptom 5]
- [symptom 6]
Physical Examination
- Patients with [disease name] usually appear [general appearance].
- Physical examination may be remarkable for:
- [finding 1]
- [finding 2]
- [finding 3]
- [finding 4]
- [finding 5]
- [finding 6]
Laboratory Findings
- There are no specific laboratory findings associated with [disease name].
- A [positive/negative] [test name] is diagnostic of [disease name].
- An [elevated/reduced] concentration of [serum/blood/urinary/CSF/other] [lab test] is diagnostic of [disease name].
- Other laboratory findings consistent with the diagnosis of [disease name] include [abnormal test 1], [abnormal test 2], and [abnormal test 3].
Imaging Findings
- There are no [imaging study] findings associated with [disease name].
- [Imaging study 1] is the imaging modality of choice for [disease name].
- On [imaging study 1], [disease name] is characterized by [finding 1], [finding 2], and [finding 3].
- [Imaging study 2] may demonstrate [finding 1], [finding 2], and [finding 3].
Other Diagnostic Studies
- [Disease name] may also be diagnosed using [diagnostic study name].
- Findings on [diagnostic study name] include [finding 1], [finding 2], and [finding 3].
Treatment
Medical Therapy
- There is no treatment for [disease name]; the mainstay of therapy is supportive care.
- The mainstay of therapy for [disease name] is [medical therapy 1] and [medical therapy 2].
- [Medical therapy 1] acts by [mechanism of action 1].
- Response to [medical therapy 1] can be monitored with [test/physical finding/imaging] every [frequency/duration].
Surgery
- Surgery is the mainstay of therapy for [disease name].
- [Surgical procedure] in conjunction with [chemotherapy/radiation] is the most common approach to the treatment of [disease name].
- [Surgical procedure] can only be performed for patients with [disease stage] [disease name].
Prevention
- There are no primary preventive measures available for [disease name].
- Effective measures for the primary prevention of [disease name] include [measure1], [measure2], and [measure3].
- Once diagnosed and successfully treated, patients with [disease name] are followed-up every [duration]. Follow-up testing includes [test 1], [test 2], and [test 3].
References
- ↑ Rosen, Samuel H.; Castleman, Benjamin; Liebow, Averill A.; Enzinger, Frank M.; Hunt, Richard T. N. (1958). "Pulmonary Alveolar Proteinosis". New England Journal of Medicine. 258 (23): 1123–1142. doi:10.1056/NEJM195806052582301. ISSN 0028-4793.
- ↑ Ramirez-R., Jose; Nyka, Walenty; McLaughlin, Joseph (1963). "Pulmonary Alveolar Proteinosis". New England Journal of Medicine. 268 (4): 165–171. doi:10.1056/NEJM196301242680401. ISSN 0028-4793.
- ↑ Suzuki T, Trapnell BC (September 2016). "Pulmonary Alveolar Proteinosis Syndrome". Clin. Chest Med. 37 (3): 431–40. doi:10.1016/j.ccm.2016.04.006. PMID 27514590.
- ↑ Brasch F, Birzele J, Ochs M, Guttentag SH, Schoch OD, Boehler A, Beers MF, Müller KM, Hawgood S, Johnen G (September 2004). "Surfactant proteins in pulmonary alveolar proteinosis in adults". Eur. Respir. J. 24 (3): 426–35. doi:10.1183/09031936.04.00076403. PMID 15358702.
- ↑ Mulugeta S, Gray JM, Notarfrancesco KL, Gonzales LW, Koval M, Feinstein SI, Ballard PL, Fisher AB, Shuman H (June 2002). "Identification of LBM180, a lamellar body limiting membrane protein of alveolar type II cells, as the ABC transporter protein ABCA3". J. Biol. Chem. 277 (25): 22147–55. doi:10.1074/jbc.M201812200. PMID 11940594.
- ↑ Salerno T, Peca D, Menchini L, Schiavino A, Petreschi F, Occasi F, Cogo P, Danhaive O, Cutrera R (March 2014). "Respiratory insufficiency in a newborn with congenital hypothyroidism due to a new mutation of TTF-1/NKX2.1 gene". Pediatr. Pulmonol. 49 (3): E42–4. doi:10.1002/ppul.22788. PMID 23997037.
- ↑ "GeneReviews® - NCBI Bookshelf".
- ↑ Hadchouel A, Wieland T, Griese M, Baruffini E, Lorenz-Depiereux B, Enaud L, Graf E, Dubus JC, Halioui-Louhaichi S, Coulomb A, Delacourt C, Eckstein G, Zarbock R, Schwarzmayr T, Cartault F, Meitinger T, Lodi T, de Blic J, Strom TM (May 2015). "Biallelic Mutations of Methionyl-tRNA Synthetase Cause a Specific Type of Pulmonary Alveolar Proteinosis Prevalent on Réunion Island". Am. J. Hum. Genet. 96 (5): 826–31. doi:10.1016/j.ajhg.2015.03.010. PMC 4570277. PMID 25913036.
Overview
Pulmonary alveolar proteinosis -(PAP) is a rare lung disease in which abnormal accumulation of surfactant occurs within the alveoli, interfering with gas exchange. PAP can occur in a primary form or secondarily in the settings of malignancy (especially in myeloid leukemia), pulmonary infection, or environmental exposure to dusts or chemicals. Rare familial forms have also been recognized, suggesting a genetic component in some cases. [1] [2] [3] [4]
Historical Perspective
Pathophysiology
Although the cause of PAP remains obscure, a major breakthrough in the understanding of the etiology of the disease came by the chance observation that mice bred for experimental study to lack a hematologic growth factor known as granulocyte-macrophage colony stimulating factor (GM-CSF) developed a pulmonary syndrome of abnormal surfactant accumulation resembling human PAP. The implications of this finding are still being explored, but significant progress was reported in February, 2007. Researchers in that report discussed the presence of anti-GM-CSF autoantibodies in patients with PAP, and duplicated that syndrome with the infusion of these autoantibodies into mice. [5]
Causes
Pulmonary alveolar proteinosis is an idiopathic disease, but studies have linked causes to production of antibodies that neutralize GM-CSF, granulocyte-macrophage colony-stimulating factor. Occasionally, development of pulmonary alveolar proteinosis is related to exposure of toxic substances, such as inorganic dusts, infection with Pneumocystis jirovecii, certain cancers, and immunosuppressants. It rarely occurs in newborns.
There are several types of clinical forms of Pulmonary alveolar proteinosis; primary, secondary, and congenital. Primary PAP involves high levels of antibodies that neutralize GM-CSF, granulocyte-macrophage colony-stimulating factor. Secondary PAP occurs from clinical conditions that reduce the numbers and functions of alveolar macrophages. Congenital PAP is due to genetic mutations in the genes encoding surfactant proteins of the receptor for granulocyte-macrophage colony-stimulating factor.[6]
Causes
Common Causes
- Pneumocystis jirovecii
- Respiratory failure
- Uncontrolled infection
- Respiratory Pathogens
- Opportunistic Pathogens: Nocardia
- Sirolimus
- Prednisone
- Inorganic dusts
- Inhalation of silica dust
- Exposure to insecticides
- aluminum dust
- titanium dioxide
- Haematological malignancies
- HIV infection.
Causes by Organ System
| Cardiovascular | No underlying causes |
| Chemical / poisoning | No underlying causes |
| Dermatologic | No underlying causes |
| Drug Side Effect | Sirolimus, Prednisone |
| Ear Nose Throat | No underlying causes |
| Endocrine | No underlying causes |
| Environmental | Inorganic dusts, Inhalation of silica dust, Exposure to insecticides, aluminum dust, titanium dioxide |
| Gastroenterologic | No underlying causes |
| Genetic | No underlying causes |
| Hematologic | Haematological malignancies |
| Iatrogenic | No underlying causes |
| Infectious Disease | Uncontrolled infection, Respiratory Pathogens, Nocardia, HIV infection |
| Musculoskeletal / Ortho | [No underlying causes |
| Neurologic | No underlying causes |
| Nutritional / Metabolic | No underlying causes |
| Obstetric/Gynecologic | No underlying causes |
| Oncologic | Haematological malignancies |
| Opthalmologic | No underlying causes |
| Overdose / Toxicity | No underlying causes |
| Psychiatric | No underlying causes |
| Pulmonary | Pneumocystis jirovecii, Respiratory failure |
| Renal / Electrolyte | No underlying causes |
| Rheum / Immune / Allergy | No underlying causes |
| Sexual | No underlying causes |
| Trauma | No underlying causes |
| Urologic | No underlying causes |
| Dental | No underlying causes |
| Miscellaneous | No underlying causes |
Causes in Alphabetical Order
|
Natural History
The clinical course of PAP is unpredictable. Spontaneous remission is recognized; some patients have stable symptoms.
Complications
Death may occur due to progression of PAP or due to the underlying disease associated with PAP. Individuals with PAP are more vulnerable to infection of the lung by bacteria or fungi.
Prognosis
History and Symptoms
The symptoms of PAP include:
Chest X Ray
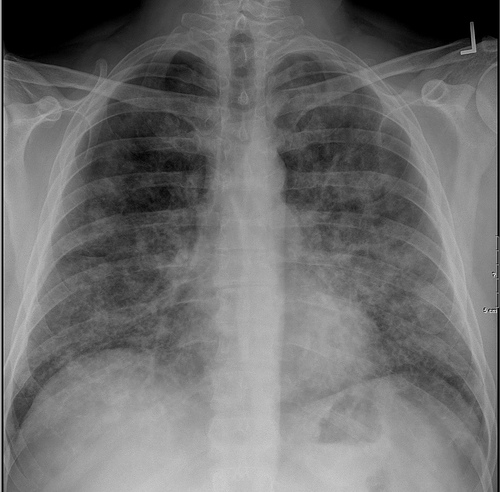
Classic radiographic finding is bilateral, symmetric alveolar consolidation or ground-glass opacity, particularly in a perihilar or hilar distribution resembling pulmonary edema.
CT
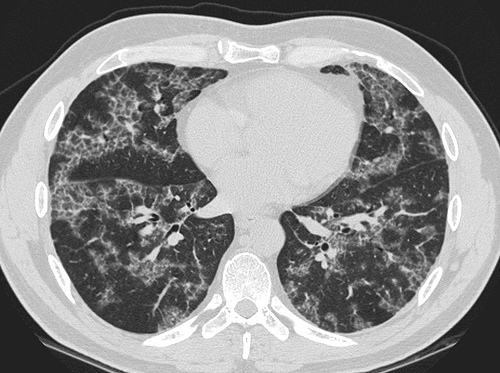
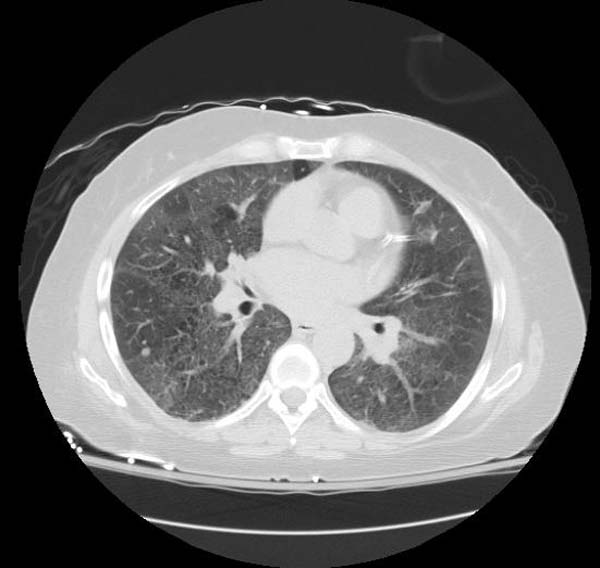
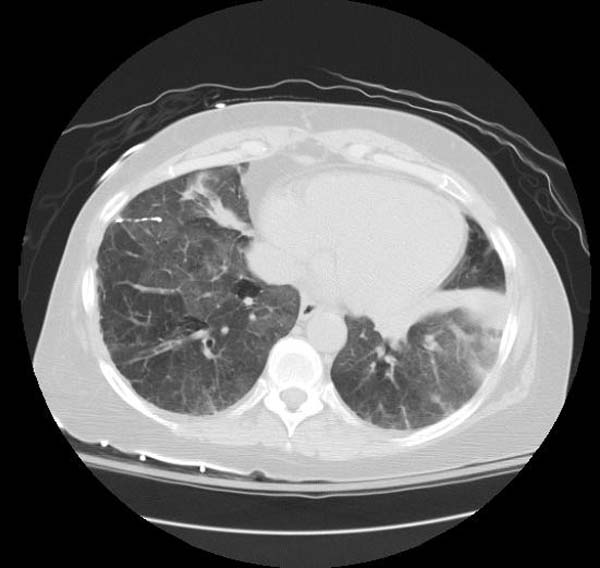
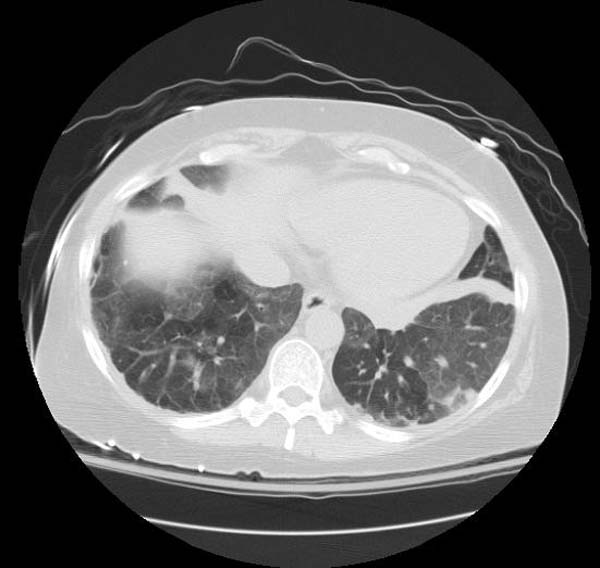
- CT typically shows diffuse ground-glass attenuation with superimposed crazy-paving pattern (intra- and interlobular septal thickening, often in polygonal shapes representing the secondary pulmonary lobule).
Patient#1
Patient #1: Proven PAP but not quite the classic crazy-paving pattern
Other Diagnostic Studies
Diagnosis is generally made by surgical or endoscopic biopsy of the lung, revealing the distinctive pathologic finding.
Histopathological Findings: Pulmonary alveolar proteinosis
{{#ev:youtube|L9fVRzcPkEQ}}
Medical Therapy
The use of GM-CSF injections has also been attempted, with variable success.
Surgery
The standard treatment for PAP is whole-lung lavage, in which sterile fluid is instilled into the lung and then removed, along with the abnormal surfactant material. This is generally effective at ameliorating symptoms, often for prolonged periods. Lung transplantation can be performed in refractory cases.
References
- ↑ Rosen SH, Castleman B, and Liebow AA. Pulmonary alveolar proteinosis. New England Journal of Medicine 1958; 258: 1123-1142.
- ↑ Seymour JF and Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. American Journal of Respiratory and Critical Care Medicine 2002; 166: 215-235.
- ↑ Shah PL, Hansell D, Lawson PR, et al. Pulmonary alveolar proteinosis; clinical aspects and current concepts on pathogenesis. Thorax 2000; 55: 67-77.
- ↑ Stanley E, Lieschke GJ, Grail D, et al. Granulocyte/macrophage colony-stimulating factor-deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc. Natl. Acad. Sci. USA 1994; 91: 5592-5596.
- ↑ Uchida K, Beck D, Yamamoto T, Berclaz P, Abe S, Staudt M, Carey B, Filippi M, Wert S, Denson L, Puchalski J, Hauck D, Trapnell B (2007). "GM-CSF autoantibodies and neutrophil dysfunction in pulmonary alveolar proteinosis". N Engl J Med. 356 (6): 567–79. PMID 17287477.
- ↑ 6.0 6.1 Trapnell BC, Whitsett JA, Nakata K (2003). "Pulmonary alveolar proteinosis". N Engl J Med. 349 (26): 2527–39. doi:10.1056/NEJMra023226. PMID 14695413.