Psoriatic arthritis: Difference between revisions
Jump to navigation
Jump to search
| (23 intermediate revisions by the same user not shown) | |||
| Line 121: | Line 121: | ||
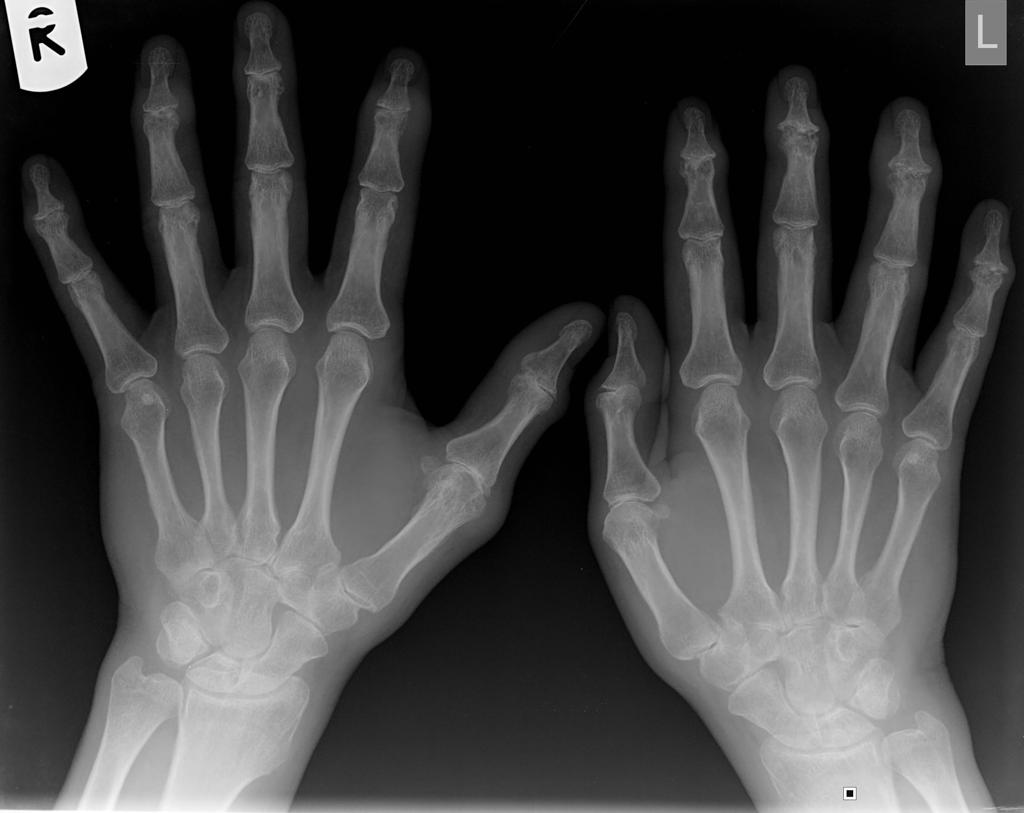
[[File:Psoriatic-arthritis of hands.jpg|centre|thumb|Psoriatic-arthritis of hands showing pencil-in-cup deformity - By Case courtesy of Dr Jeremy Jones, <a href="https://radiopaedia.org/">Radiopaedia.org</a>. From the case <a href="https://radiopaedia.org/cases/8798">rID: 8798</a>]] | [[File:Psoriatic-arthritis of hands.jpg|centre|thumb|Psoriatic-arthritis of hands showing pencil-in-cup deformity - By Case courtesy of Dr Jeremy Jones, <a href="https://radiopaedia.org/">Radiopaedia.org</a>. From the case <a href="https://radiopaedia.org/cases/8798">rID: 8798</a>]] | ||
*MRI: | * MRI: MRI may reveal the following findings:<ref name="pmid20966327">{{cite journal |vauthors=Spira D, Kötter I, Henes J, Kümmerle-Deschner J, Schulze M, Boss A, Horger M |title=MRI findings in psoriatic arthritis of the hands |journal=AJR Am J Roentgenol |volume=195 |issue=5 |pages=1187–93 |date=November 2010 |pmid=20966327 |doi=10.2214/AJR.10.4281 |url=}}</ref> | ||
** [[enthesitis]] | |||
** [[Periostitis]] | |||
** Joint erosions | |||
** Synovitis (articular or flexor tendon sheath) | |||
** Ankylosis | |||
** Bone marrow edema | |||
* Ulrasonography: Ultrasonography may reveal following findings.<ref name="pmid10451072">{{cite journal |vauthors=Kane D, Greaney T, Bresnihan B, Gibney R, FitzGerald O |title=Ultrasonography in the diagnosis and management of psoriatic dactylitis |journal=J. Rheumatol. |volume=26 |issue=8 |pages=1746–51 |date=August 1999 |pmid=10451072 |doi= |url=}}</ref> | |||
** Joint effusions and widening of joint space | |||
** Synovitis (articular and flexor tenosynovitis) | |||
** Dactylitis | |||
** Thickening of the joint capsule | |||
=== Other Diagnostic Studies === | === Other Diagnostic Studies === | ||
| Line 155: | Line 166: | ||
** '''Severe disease''' '''and the presence of adverse prognostic factors''': [[TNF inhibitor|TNF inhibitors]] (eg, [[etanercept]], [[infliximab]], [[adalimumab]], [[golimumab]]), interleukin(IL) inhibitors (eg, [[secukinumab]], [[ixekizumab]], [[abatacept]]). | ** '''Severe disease''' '''and the presence of adverse prognostic factors''': [[TNF inhibitor|TNF inhibitors]] (eg, [[etanercept]], [[infliximab]], [[adalimumab]], [[golimumab]]), interleukin(IL) inhibitors (eg, [[secukinumab]], [[ixekizumab]], [[abatacept]]). | ||
*** Biologic DMARDs are considered for patients who fail to respond or contraindication to conventional synthetic DMARDs. It is also administered in patients with presence of poor prognosis factors, even if they have not failed a standard DMARDs therapy. | *** Biologic DMARDs are considered for patients who fail to respond or contraindication to conventional synthetic DMARDs. It is also administered in patients with presence of poor prognosis factors, even if they have not failed a standard DMARDs therapy. | ||
**** TNF inhibitors: | |||
***** Preferred regimen (1): [[Adalimumab]]<ref name="pmid16200601">{{cite journal |vauthors=Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, Sharp JT, Ory PA, Perdok RJ, Weinberg MA |title=Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial |journal=Arthritis Rheum. |volume=52 |issue=10 |pages=3279–89 |date=October 2005 |pmid=16200601 |doi=10.1002/art.21306 |url=}}</ref>: It is a human anti-[[Tumor necrosis factor-alpha|TNF alpha]] monoclonal [[antibody]]. Dosage: 40 mg can be given s.c every 2 weeks | ***** Preferred regimen (1): [[Adalimumab]]<ref name="pmid16200601">{{cite journal |vauthors=Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, Sharp JT, Ory PA, Perdok RJ, Weinberg MA |title=Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial |journal=Arthritis Rheum. |volume=52 |issue=10 |pages=3279–89 |date=October 2005 |pmid=16200601 |doi=10.1002/art.21306 |url=}}</ref>: It is a human anti-[[Tumor necrosis factor-alpha|TNF alpha]] monoclonal [[antibody]]. Dosage: 40 mg can be given s.c every 2 weeks | ||
***** Preferred regimen (2): [[Etanercept]]<ref name="pmid15248226">{{cite journal |vauthors=Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, Salonen D, Rubenstein J, Sharp JT, Tsuji W |title=Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression |journal=Arthritis Rheum. |volume=50 |issue=7 |pages=2264–72 |date=July 2004 |pmid=15248226 |doi=10.1002/art.20335 |url=}}</ref>: It is a [[Tumor necrosis factors|TNF]] receptor p75-IgG1 fusion [[protein]]. Dosage: 50 mg can be given s.c every week. | ***** Preferred regimen (2): [[Etanercept]]<ref name="pmid15248226">{{cite journal |vauthors=Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, Salonen D, Rubenstein J, Sharp JT, Tsuji W |title=Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression |journal=Arthritis Rheum. |volume=50 |issue=7 |pages=2264–72 |date=July 2004 |pmid=15248226 |doi=10.1002/art.20335 |url=}}</ref>: It is a [[Tumor necrosis factors|TNF]] receptor p75-IgG1 fusion [[protein]]. Dosage: 50 mg can be given s.c every week. | ||
| Line 161: | Line 172: | ||
***** Preferred regimen (4): [[Golimumab]]<ref name="pmid19333944">{{cite journal |vauthors=Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, Papp K, Zrubek J, Mudivarthy S, Mack M, Visvanathan S, Beutler A |title=Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled study |journal=Arthritis Rheum. |volume=60 |issue=4 |pages=976–86 |date=April 2009 |pmid=19333944 |doi=10.1002/art.24403 |url=}}</ref>: it is a human IgG1k anti-[[Tumor necrosis factor-alpha|TNF alpha antibody]]. Dosage: 50 mg can be given s.c and monthly. | ***** Preferred regimen (4): [[Golimumab]]<ref name="pmid19333944">{{cite journal |vauthors=Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, Papp K, Zrubek J, Mudivarthy S, Mack M, Visvanathan S, Beutler A |title=Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled study |journal=Arthritis Rheum. |volume=60 |issue=4 |pages=976–86 |date=April 2009 |pmid=19333944 |doi=10.1002/art.24403 |url=}}</ref>: it is a human IgG1k anti-[[Tumor necrosis factor-alpha|TNF alpha antibody]]. Dosage: 50 mg can be given s.c and monthly. | ||
***** Preferred regimen (5): [[Certolizumab pegol]]<ref name="pmid23942868">{{cite journal |vauthors=Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, Woltering F, Stach C, Hoepken B, Arledge T, van der Heijde D |title=Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA) |journal=Ann. Rheum. Dis. |volume=73 |issue=1 |pages=48–55 |date=January 2014 |pmid=23942868 |pmc=3888622 |doi=10.1136/annrheumdis-2013-203696 |url=}}</ref>: It is a Fab fragment of anti-[[Tumor necrosis factor-alpha|TNF alpha monoclonal antibody]]. Dosage: 400 mg at 0, 2, and 4 weeks can be given s.c and then 200 mg every 2 weeks sub cutaneously. | ***** Preferred regimen (5): [[Certolizumab pegol]]<ref name="pmid23942868">{{cite journal |vauthors=Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, Woltering F, Stach C, Hoepken B, Arledge T, van der Heijde D |title=Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA) |journal=Ann. Rheum. Dis. |volume=73 |issue=1 |pages=48–55 |date=January 2014 |pmid=23942868 |pmc=3888622 |doi=10.1136/annrheumdis-2013-203696 |url=}}</ref>: It is a Fab fragment of anti-[[Tumor necrosis factor-alpha|TNF alpha monoclonal antibody]]. Dosage: 400 mg at 0, 2, and 4 weeks can be given s.c and then 200 mg every 2 weeks sub cutaneously. | ||
****** Adverse effects: Reactivation of latent Tuberculosis, increased risk for infections including bacterial and opportunistic infection. Therefore, before starting TNF inhibitors screening for TB, Hepatitis B, and C | ****** Adverse effects: Reactivation of latent Tuberculosis, increased risk for infections including bacterial and opportunistic infection. Therefore, before starting treatment with TNF inhibitors screening for TB, Hepatitis B, and C should be done. | ||
**** IL inhibitor therapy: This is considered in patients with severe peripheral arthritis, where TNF therapy is contraindicated or not responding even after switching to different TNF inhibitor. | |||
***** Anti-IL-17 therapies : | |||
****** Preferred regimen (1): Secukinumab<ref name="pmid26135703">{{cite journal |vauthors=McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, van der Heijde D, Landewé R, Conaghan PG, Gottlieb AB, Richards H, Pricop L, Ligozio G, Patekar M, Mpofu S |title=Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial |journal=Lancet |volume=386 |issue=9999 |pages=1137–46 |date=September 2015 |pmid=26135703 |doi=10.1016/S0140-6736(15)61134-5 |url=}}</ref>: It is a monoclonal antibody against IL-17A. Dosage: 150 mg at weeks 0, 1, 2, 3, and 4 and then every 4 weeks can be given subcutaneously. | |||
****** Preferred regimen (2): Ixekizumab<ref name="pmid27553214">{{cite journal |vauthors=Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, Lin CY, Braun DK, Lee CH, Gladman DD |title=Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1 |journal=Ann. Rheum. Dis. |volume=76 |issue=1 |pages=79–87 |date=January 2017 |pmid=27553214 |pmc=5264219 |doi=10.1136/annrheumdis-2016-209709 |url=}}</ref> : Dosage: 160 mg initially, then 80 mg every two or four weeks can be given subcutaneously. | |||
***** Anti-IL-12/23 therapy: | |||
****** Ustekinumab<ref name="pmid23769296">{{cite journal |vauthors=McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, Brodmerkel C, Li S, Wang Y, Mendelsohn AM, Doyle MK |title=Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial |journal=Lancet |volume=382 |issue=9894 |pages=780–9 |date=August 2013 |pmid=23769296 |doi=10.1016/S0140-6736(13)60594-2 |url=}}</ref>: It is a IgG1 monoclonal antibody against the shared P40 subunit of human IL-12 and IL-23. Dosage: 45 mg at weeks 0 and 4 and then for every 12 weeks can be given subcutaneously. | |||
**** Abatacept<ref name="pmid28473423">{{cite journal |vauthors=Mease PJ, Gottlieb AB, van der Heijde D, FitzGerald O, Johnsen A, Nys M, Banerjee S, Gladman DD |title=Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis |journal=Ann. Rheum. Dis. |volume=76 |issue=9 |pages=1550–1558 |date=September 2017 |pmid=28473423 |pmc=5561378 |doi=10.1136/annrheumdis-2016-210724 |url=}}</ref>: It is a costimulatory T-cell molecule, blocking signal activation of the CD28 receptor on the T cell inhibiting T-cell activation. Dosage: 125 mg can be given subcutaneously once in a week. | |||
* '''Axial disease/ spondylitis''':<ref name="pmid25362712">{{cite journal |vauthors=Nash P, Lubrano E, Cauli A, Taylor WJ, Olivieri I, Gladman DD |title=Updated guidelines for the management of axial disease in psoriatic arthritis |journal=J. Rheumatol. |volume=41 |issue=11 |pages=2286–9 |date=November 2014 |pmid=25362712 |doi=10.3899/jrheum.140877 |url=}}</ref> | |||
** Mild disease: Non-steroidal anti-inflammatory drugs (NSAIDs), local corticosteroid injections, patient education, exercise and physiotherapy may be recommended to treat mild axial involvement. | |||
*** Preferred regimen (1): [[Naproxen sodium|Naproxen]]: 375-500 mg/twice a day | |||
*** Preferred regimen (2): [[Celecoxib]]: 200 mg/twice a day | |||
** Moderate to severe disease: TNF inhibitors | |||
* [[Skin disease|'''Skin disease''']]:<ref name="pmid24566842">{{cite journal |vauthors=Mease PJ, Armstrong AW |title=Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis |journal=Drugs |volume=74 |issue=4 |pages=423–41 |date=March 2014 |pmid=24566842 |pmc=3958815 |doi=10.1007/s40265-014-0191-y |url=}}</ref> | |||
** Phototherapy: First line of treatment including UVB, PUVA. | |||
** Fumeric esters, retinols, calcipotriol | |||
** Conventional DMARDs and TNF inhibitors, and retinoic acid derivatives (eg, acitretin) may be used in combinatination with phototherapy. | |||
* [[Nail changes|'''Nail disease''']]:<ref name="pmid25471223">{{cite journal |vauthors=Crowley JJ, Weinberg JM, Wu JJ, Robertson AD, Van Voorhees AS |title=Treatment of nail psoriasis: best practice recommendations from the Medical Board of the National Psoriasis Foundation |journal=JAMA Dermatol |volume=151 |issue=1 |pages=87–94 |date=January 2015 |pmid=25471223 |doi=10.1001/jamadermatol.2014.2983 |url=}}</ref> | |||
** Topical corticosteroids, calcipotriol creams, DMARDs and TNF inhibitors may be helpful. | |||
* '''Enthesitis''': | |||
** Mild disease: NSAIDs, local corticosteroid injection, phycial therapy may be helpful. | |||
** Moderate to severe disease: TNF inhibitors | |||
* [[Dactylitis|'''Dactylitis''']]:<ref name="pmid25362714">{{cite journal |vauthors=Rose S, Toloza S, Bautista-Molano W, Helliwell PS |title=Comprehensive treatment of dactylitis in psoriatic arthritis |journal=J. Rheumatol. |volume=41 |issue=11 |pages=2295–300 |date=November 2014 |pmid=25362714 |doi=10.3899/jrheum.140879 |url=}}</ref> | |||
** NSAIDs, steroid injections, conventional DMARDs and TNF inhibitors can be helpful. | |||
** Biologic DMARDs can be considered for treatment of dactylitis if, therapy with NSAIDs, steroid injections, conventional DMARDs fails. | |||
* '''Non pharmacologic therapy''': | * '''Non pharmacologic therapy''': | ||
** [[Physical exercise|Exercise]] | ** [[Physical exercise|Exercise]] | ||
| Line 170: | Line 205: | ||
** Educating the [[patient]] about [[disease]] course, [[joint]] protection and [[Comorbidity|comorbid]] conditions. | ** Educating the [[patient]] about [[disease]] course, [[joint]] protection and [[Comorbidity|comorbid]] conditions. | ||
** [[Orthotics]] | ** [[Orthotics]] | ||
=== Surgery === | === Surgery === | ||
*Surgery | *Surgery may be indicated in patients of psoriatic arthritis with severe joint damage that limit mobility.<ref name="pmid10782824">{{cite journal |vauthors=Zangger P, Esufali ZH, Gladman DD, Bogoch ER |title=Type and outcome of reconstructive surgery for different patterns of psoriatic arthritis |journal=J. Rheumatol. |volume=27 |issue=4 |pages=967–74 |date=April 2000 |pmid=10782824 |doi= |url=}}</ref> | ||
*Common procedures include hand joint surgery involving PIP and DIP joints, hip or knee surgery. | |||
* | |||
=== Prevention === | === Prevention === | ||
*There are no primary preventive measures | * There are no established primary preventive measures for psoriatic arthritis. | ||
* Patients are monitored regularly for disease activity, drug efficacy, adverse effects and associated comorbid conditions. | |||
* | |||
==References== | ==References== | ||
Revision as of 20:08, 18 April 2018
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Chandrakala Yannam, MD [2]
Overview
Historical Perspective
- [Disease name] was first discovered by [scientist name], a [nationality + occupation], in [year] during/following [event].
- In [year], [gene] mutations were first identified in the pathogenesis of [disease name].
- In [year], the first [discovery] was developed by [scientist] to treat/diagnose [disease name].
Classification
- [Disease name] may be classified according to [classification method] into [number] subtypes/groups:
- [group1]
- [group2]
- [group3]
- Other variants of [disease name] include [disease subtype 1], [disease subtype 2], and [disease subtype 3].
Pathophysiology
- The pathogenesis of [disease name] is characterized by [feature1], [feature2], and [feature3].
- The [gene name] gene/Mutation in [gene name] has been associated with the development of [disease name], involving the [molecular pathway] pathway.
- On gross pathology, [feature1], [feature2], and [feature3] are characteristic findings of [disease name].
- On microscopic histopathological analysis, [feature1], [feature2], and [feature3] are characteristic findings of [disease name].
Clinical Features
Differentiating [disease name] from other Diseases
- [Disease name] must be differentiated from other diseases that cause [clinical feature 1], [clinical feature 2], and [clinical feature 3], such as:
- [Differential dx1]
- [Differential dx2]
- [Differential dx3]
Epidemiology and Demographics
- The prevalence of [disease name] is approximately [number or range] per 100,000 individuals worldwide.
- In [year], the incidence of [disease name] was estimated to be [number or range] cases per 100,000 individuals in [location].
Age
- Patients of all age groups may develop [disease name].
- [Disease name] is more commonly observed among patients aged [age range] years old.
- [Disease name] is more commonly observed among [elderly patients/young patients/children].
Gender
- In general, there is no gender predilection to psoriatic arthritis.
Race
- There is no racial predilection for [disease name].
- [Disease name] usually affects individuals of the [race 1] race.
- [Race 2] individuals are less likely to develop [disease name].
Risk Factors
- Common risk factors in the development of [disease name] are [risk factor 1], [risk factor 2], [risk factor 3], and [risk factor 4].
Natural History, Complications and Prognosis
- The majority of patients with [disease name] remain asymptomatic for [duration/years].
- Early clinical features include [manifestation 1], [manifestation 2], and [manifestation 3].
- If left untreated, [#%] of patients with [disease name] may progress to develop [manifestation 1], [manifestation 2], and [manifestation 3].
- Common complications of [disease name] include [complication 1], [complication 2], and [complication 3].
- Prognosis is generally [excellent/good/poor], and the [1/5/10year mortality/survival rate] of patients with [disease name] is approximately [#%].
Diagnosis
Diagnostic Criteria
- The diagnosis of [disease name] is made when at least [number] of the following [number] diagnostic criteria are met:
- [criterion 1]
- [criterion 2]
- [criterion 3]
- [criterion 4]
Symptoms
- [Disease name] is usually asymptomatic.
- Symptoms of [disease name] may include the following:
- [symptom 1]
- [symptom 2]
- [symptom 3]
- [symptom 4]
- [symptom 5]
- [symptom 6]
Physical Examination
- Patients with [disease name] usually appear [general appearance].
- Physical examination may be remarkable for:
- [finding 1]
- [finding 2]
- [finding 3]
- [finding 4]
- [finding 5]
- [finding 6]
Laboratory Findings
- There are no specific laboratory findings associated with psoriatic arthritis and most the tests are non-specific.
- However, there are certain laboratory tests that can check for markers of inflammation and to exclude other diseases. These include:[1]
- CBC with differential count
- Elevated ESR
- Elevated CRP (C- reactive protein)
- Autoantibodies: The following autoantibodies may be found in patients with psoriatic arthritis.[2]
- Rheumatoid factor
- ANA (Antinuclear antibodies)
- Anti-citrullinated peptide antibodies (ACPA)
- Genetic markers:[3][4]
- Synovial fluid analysis: Elevated WBC count suggestive of inflammation.
Imaging Findings
- X-ray of digits:[5][6][7]
- Bone destructive changes including formation of subchondral cyst and erosions
- Fluffy periostitis
- Ankylosis
- Phalangeal tuft acroosteolysis
- New bone formation: Perisoteal and endosteal bone formation may result in increased bone density of an entire phalanx resulting in so called ivory phalanx.
- Pencil-in-cup deformity (osteolytic lesions) usually involving DIP joints but also affects PIP joints
- Osteolysis and ankylosis both coexists in the same joints of hands and foot
- Enthesitis
- Dactylitis (sausage digit)
- Gross finger deformity
- Arthritis mutilans: It may lead to "telescoping of fingers" caused by marked bony resorption and the subsequent collapse of soft tissue
- Asymmetrical sacroiliitis
- Spondylitis: Asymmetric paravertebral ossifications and relative sparing of the facet joints
- MRI: MRI may reveal the following findings:[8]
- enthesitis
- Periostitis
- Joint erosions
- Synovitis (articular or flexor tendon sheath)
- Ankylosis
- Bone marrow edema
- Ulrasonography: Ultrasonography may reveal following findings.[9]
- Joint effusions and widening of joint space
- Synovitis (articular and flexor tenosynovitis)
- Dactylitis
- Thickening of the joint capsule
Other Diagnostic Studies
- Bone mineral density (BMD) testing: Bone density may be decreased in psoriatic arthritis resulting in osteoporosis and increased risk for fractures. [10]
Treatment
Medical Therapy
- Medical therapy for psoriatic arthritis is according to the guidelines proposed by
- European League Against Rheumatism (EULAR): Guidelines were first proposed in 2012 and they were updated in 2015.[11][12]
- Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA)[13]
- American College of Rheumatology (ACR)
- National Psoriasis Foundation (NPF)
- American Academy of Dermatology (AAD) Psoriasis Guidelines of Care[14]
- British Society of Rheumatology (BSR)[15]
- Pharmacologic therapy for psoriatic arthritis include, non-steroidal anti-inflammatory drugs (NSAIDs), conventional synthetic DMARDs (eg, methotrexate, sulfasalazine, cyclosporin A, leflunomide), biologicDMARDs including, TNF inhibitors (eg, etanercept, infliximab, adalimumab, golimumab), phosphodiesterase (PDE) inhibitors (eg, apremilast), interleukin(IL) inhibitors (eg, secukinumab, ixekizumab, abatacept) and intraarticular glucocorticoid injections.
- Peripheral arthritis:
- Mild disease: Nonsteroidal antiinflammatory drugs (NSAIDs) are the most commonly used drugs for the management of mild active psoriatic arthritis.[16][17]
- Preferred regimen (1): Naproxen: 375-500 mg/twice a day
- Preferred regimen (2): Celecoxib: 200 mg/twice a day
- Preferred regimen (3): Nimesulide: 200 and 400 mg/day
- Preferred regimen (4): Ibuprofen: Max dose of up to 2400 mg/day
- Adverse effects of non-steroidal anti-inflammatory drugs include increased cardiovascular risk, gastritis, ulcers and low renal clearance.
- Moderate to severe disease: Conventional synthetic disease-modifying antirheumatic drugs (DMARDs) may be considered in patients with moderate to severe active peripheral arthritis. These are also considered in patients who are resistant or not responding to NSAIDs, and local corticosteroid injections.
- Preferred regimen (1): Methotrexate[18][19]: 15 to 25 mg/week
- Adverse effects of methotrexate: Liver toxicity, immunosuppression, interstitial pneumonitis, increased infection risk.
- Folic acid supplementation should be given to all patients taking methotrexate.
- Preferred regimen (2): Leflunomide: 20 mg/day
- Adverse effects of Leflunomide: Liver toxicity, diarrhea, rash, alopecia, pneumonitis.
- Preferred regimen (3): Sulfasalazine[19]: 2-3 gms/day
- Adverse effects of sulfasalazine: Nausea, diarrhea, abdominal pain, rash, and neutropenia.
- Preferred regimen (4): Cyclosporine A: 3.5 mg/kg per day[20]
- Preferred regimen (1): Methotrexate[18][19]: 15 to 25 mg/week
- Severe disease and the presence of adverse prognostic factors: TNF inhibitors (eg, etanercept, infliximab, adalimumab, golimumab), interleukin(IL) inhibitors (eg, secukinumab, ixekizumab, abatacept).
- Biologic DMARDs are considered for patients who fail to respond or contraindication to conventional synthetic DMARDs. It is also administered in patients with presence of poor prognosis factors, even if they have not failed a standard DMARDs therapy.
- TNF inhibitors:
- Preferred regimen (1): Adalimumab[21]: It is a human anti-TNF alpha monoclonal antibody. Dosage: 40 mg can be given s.c every 2 weeks
- Preferred regimen (2): Etanercept[22]: It is a TNF receptor p75-IgG1 fusion protein. Dosage: 50 mg can be given s.c every week.
- Preferred regimen (3): Infliximab[23]: It is a chimeric monoclonal antibody against TNF alpha. Dosage: 5 mg/kg at weeks 0, 2, and 6 and after that 5 mg/kg every 6-8 weeks.
- Preferred regimen (4): Golimumab[24]: it is a human IgG1k anti-TNF alpha antibody. Dosage: 50 mg can be given s.c and monthly.
- Preferred regimen (5): Certolizumab pegol[25]: It is a Fab fragment of anti-TNF alpha monoclonal antibody. Dosage: 400 mg at 0, 2, and 4 weeks can be given s.c and then 200 mg every 2 weeks sub cutaneously.
- Adverse effects: Reactivation of latent Tuberculosis, increased risk for infections including bacterial and opportunistic infection. Therefore, before starting treatment with TNF inhibitors screening for TB, Hepatitis B, and C should be done.
- IL inhibitor therapy: This is considered in patients with severe peripheral arthritis, where TNF therapy is contraindicated or not responding even after switching to different TNF inhibitor.
- Anti-IL-17 therapies :
- Preferred regimen (1): Secukinumab[26]: It is a monoclonal antibody against IL-17A. Dosage: 150 mg at weeks 0, 1, 2, 3, and 4 and then every 4 weeks can be given subcutaneously.
- Preferred regimen (2): Ixekizumab[27] : Dosage: 160 mg initially, then 80 mg every two or four weeks can be given subcutaneously.
- Anti-IL-12/23 therapy:
- Ustekinumab[28]: It is a IgG1 monoclonal antibody against the shared P40 subunit of human IL-12 and IL-23. Dosage: 45 mg at weeks 0 and 4 and then for every 12 weeks can be given subcutaneously.
- Anti-IL-17 therapies :
- Abatacept[29]: It is a costimulatory T-cell molecule, blocking signal activation of the CD28 receptor on the T cell inhibiting T-cell activation. Dosage: 125 mg can be given subcutaneously once in a week.
- TNF inhibitors:
- Biologic DMARDs are considered for patients who fail to respond or contraindication to conventional synthetic DMARDs. It is also administered in patients with presence of poor prognosis factors, even if they have not failed a standard DMARDs therapy.
- Mild disease: Nonsteroidal antiinflammatory drugs (NSAIDs) are the most commonly used drugs for the management of mild active psoriatic arthritis.[16][17]
- Axial disease/ spondylitis:[30]
- Mild disease: Non-steroidal anti-inflammatory drugs (NSAIDs), local corticosteroid injections, patient education, exercise and physiotherapy may be recommended to treat mild axial involvement.
- Moderate to severe disease: TNF inhibitors
- Skin disease:[31]
- Phototherapy: First line of treatment including UVB, PUVA.
- Fumeric esters, retinols, calcipotriol
- Conventional DMARDs and TNF inhibitors, and retinoic acid derivatives (eg, acitretin) may be used in combinatination with phototherapy.
- Nail disease:[32]
- Topical corticosteroids, calcipotriol creams, DMARDs and TNF inhibitors may be helpful.
- Enthesitis:
- Mild disease: NSAIDs, local corticosteroid injection, phycial therapy may be helpful.
- Moderate to severe disease: TNF inhibitors
- Dactylitis:[33]
- NSAIDs, steroid injections, conventional DMARDs and TNF inhibitors can be helpful.
- Biologic DMARDs can be considered for treatment of dactylitis if, therapy with NSAIDs, steroid injections, conventional DMARDs fails.
- Non pharmacologic therapy:
- Exercise
- Weight reduction
- Physical therapy
- Occupational therapy
- Educating the patient about disease course, joint protection and comorbid conditions.
- Orthotics
Surgery
- Surgery may be indicated in patients of psoriatic arthritis with severe joint damage that limit mobility.[34]
- Common procedures include hand joint surgery involving PIP and DIP joints, hip or knee surgery.
Prevention
- There are no established primary preventive measures for psoriatic arthritis.
- Patients are monitored regularly for disease activity, drug efficacy, adverse effects and associated comorbid conditions.
References
- ↑ Punzi L, Podswiadek M, Oliviero F, Lonigro A, Modesti V, Ramonda R, Todesco S (2007). "Laboratory findings in psoriatic arthritis". Reumatismo. 59 Suppl 1: 52–5. PMID 17828345.
- ↑ Johnson SR, Schentag CT, Gladman DD (May 2005). "Autoantibodies in biological agent naive patients with psoriatic arthritis". Ann. Rheum. Dis. 64 (5): 770–2. doi:10.1136/ard.2004.031286. PMC 1755477. PMID 15834057.
- ↑ Chandran V, Bull SB, Pellett FJ, Ayearst R, Rahman P, Gladman DD (October 2013). "Human leukocyte antigen alleles and susceptibility to psoriatic arthritis". Hum. Immunol. 74 (10): 1333–8. doi:10.1016/j.humimm.2013.07.014. PMID 23916976.
- ↑ Eder L, Chandran V, Pellet F, Shanmugarajah S, Rosen CF, Bull SB, Gladman DD (January 2012). "Human leucocyte antigen risk alleles for psoriatic arthritis among patients with psoriasis". Ann. Rheum. Dis. 71 (1): 50–5. doi:10.1136/ard.2011.155044. PMID 21900282.
- ↑ McGonagle D, Hermann KG, Tan AL (January 2015). "Differentiation between osteoarthritis and psoriatic arthritis: implications for pathogenesis and treatment in the biologic therapy era". Rheumatology (Oxford). 54 (1): 29–38. doi:10.1093/rheumatology/keu328. PMC 4269795. PMID 25231177.
- ↑ Siannis F, Farewell VT, Cook RJ, Schentag CT, Gladman DD (April 2006). "Clinical and radiological damage in psoriatic arthritis". Ann. Rheum. Dis. 65 (4): 478–81. doi:10.1136/ard.2005.039826. PMC 1798082. PMID 16126794.
- ↑ Haddad A, Chandran V (2013). "Arthritis mutilans". Curr Rheumatol Rep. 15 (4): 321. doi:10.1007/s11926-013-0321-7. PMID 23430715.
- ↑ Spira D, Kötter I, Henes J, Kümmerle-Deschner J, Schulze M, Boss A, Horger M (November 2010). "MRI findings in psoriatic arthritis of the hands". AJR Am J Roentgenol. 195 (5): 1187–93. doi:10.2214/AJR.10.4281. PMID 20966327.
- ↑ Kane D, Greaney T, Bresnihan B, Gibney R, FitzGerald O (August 1999). "Ultrasonography in the diagnosis and management of psoriatic dactylitis". J. Rheumatol. 26 (8): 1746–51. PMID 10451072.
- ↑ Frediani B, Allegri A, Falsetti P, Storri L, Bisogno S, Baldi F, Filipponi P, Marcolongo R (January 2001). "Bone mineral density in patients with psoriatic arthritis". J. Rheumatol. 28 (1): 138–43. PMID 11196516.
- ↑ Gossec L, Smolen JS, Ramiro S, de Wit M, Cutolo M, Dougados M, Emery P, Landewé R, Oliver S, Aletaha D, Betteridge N, Braun J, Burmester G, Cañete JD, Damjanov N, FitzGerald O, Haglund E, Helliwell P, Kvien TK, Lories R, Luger T, Maccarone M, Marzo-Ortega H, McGonagle D, McInnes IB, Olivieri I, Pavelka K, Schett G, Sieper J, van den Bosch F, Veale DJ, Wollenhaupt J, Zink A, van der Heijde D (March 2016). "European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update". Ann. Rheum. Dis. 75 (3): 499–510. doi:10.1136/annrheumdis-2015-208337. PMID 26644232.
- ↑ Gossec L, Smolen JS, Gaujoux-Viala C, Ash Z, Marzo-Ortega H, van der Heijde D, FitzGerald O, Aletaha D, Balint P, Boumpas D, Braun J, Breedveld FC, Burmester G, Cañete JD, de Wit M, Dagfinrud H, de Vlam K, Dougados M, Helliwell P, Kavanaugh A, Kvien TK, Landewé R, Luger T, Maccarone M, McGonagle D, McHugh N, McInnes IB, Ritchlin C, Sieper J, Tak PP, Valesini G, Vencovsky J, Winthrop KL, Zink A, Emery P (January 2012). "European League Against Rheumatism recommendations for the management of psoriatic arthritis with pharmacological therapies". Ann. Rheum. Dis. 71 (1): 4–12. doi:10.1136/annrheumdis-2011-200350. PMID 21953336.
- ↑ Ritchlin CT, Kavanaugh A, Gladman DD, Mease PJ, Helliwell P, Boehncke WH, de Vlam K, Fiorentino D, Fitzgerald O, Gottlieb AB, McHugh NJ, Nash P, Qureshi AA, Soriano ER, Taylor WJ (September 2009). "Treatment recommendations for psoriatic arthritis". Ann. Rheum. Dis. 68 (9): 1387–94. doi:10.1136/ard.2008.094946. PMC 2719080. PMID 18952643.
- ↑ Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, Gottlieb A, Koo JY, Lebwohl M, Leonardi CL, Lim HW, Van Voorhees AS, Beutner KR, Ryan C, Bhushan R (July 2011). "Guidelines of care for the management of psoriasis and psoriatic arthritis: section 6. Guidelines of care for the treatment of psoriasis and psoriatic arthritis: case-based presentations and evidence-based conclusions". J. Am. Acad. Dermatol. 65 (1): 137–74. doi:10.1016/j.jaad.2010.11.055. PMID 21306785.
- ↑ Coates LC, Tillett W, Chandler D, Helliwell PS, Korendowych E, Kyle S, McInnes IB, Oliver S, Ormerod A, Smith C, Symmons D, Waldron N, McHugh NJ (October 2013). "The 2012 BSR and BHPR guideline for the treatment of psoriatic arthritis with biologics". Rheumatology (Oxford). 52 (10): 1754–7. doi:10.1093/rheumatology/ket187. PMID 23887065.
- ↑ Nash P, Clegg DO (March 2005). "Psoriatic arthritis therapy: NSAIDs and traditional DMARDs". Ann. Rheum. Dis. 64 Suppl 2: ii74–7. doi:10.1136/ard.2004.030783. PMC 1766880. PMID 15708943.
- ↑ Sarzi-Puttini P, Santandrea S, Boccassini L, Panni B, Caruso I (2001). "The role of NSAIDs in psoriatic arthritis: evidence from a controlled study with nimesulide". Clin. Exp. Rheumatol. 19 (1 Suppl 22): S17–20. PMID 11296544.
- ↑ Mease P (2013). "Methotrexate in psoriatic arthritis". Bull Hosp Jt Dis (2013). 71 Suppl 1: S41–5. PMID 24219040.
- ↑ 19.0 19.1 Singh YN, Verma KK, Kumar A, Malaviya AN (November 1994). "Methotrexate in psoriatic arthritis". J Assoc Physicians India. 42 (11): 860–2. PMID 7868484.
- ↑ Steinsson K, Jónsdóttir I, Valdimarsson H (August 1990). "Cyclosporin A in psoriatic arthritis: an open study". Ann. Rheum. Dis. 49 (8): 603–6. PMC 1004173. PMID 2396865.
- ↑ Mease PJ, Gladman DD, Ritchlin CT, Ruderman EM, Steinfeld SD, Choy EH, Sharp JT, Ory PA, Perdok RJ, Weinberg MA (October 2005). "Adalimumab for the treatment of patients with moderately to severely active psoriatic arthritis: results of a double-blind, randomized, placebo-controlled trial". Arthritis Rheum. 52 (10): 3279–89. doi:10.1002/art.21306. PMID 16200601.
- ↑ Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, Salonen D, Rubenstein J, Sharp JT, Tsuji W (July 2004). "Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression". Arthritis Rheum. 50 (7): 2264–72. doi:10.1002/art.20335. PMID 15248226.
- ↑ Antoni C, Krueger GG, de Vlam K, Birbara C, Beutler A, Guzzo C, Zhou B, Dooley LT, Kavanaugh A (August 2005). "Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial". Ann. Rheum. Dis. 64 (8): 1150–7. doi:10.1136/ard.2004.032268. PMC 1755609. PMID 15677701.
- ↑ Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, Papp K, Zrubek J, Mudivarthy S, Mack M, Visvanathan S, Beutler A (April 2009). "Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: Twenty-four-week efficacy and safety results of a randomized, placebo-controlled study". Arthritis Rheum. 60 (4): 976–86. doi:10.1002/art.24403. PMID 19333944.
- ↑ Mease PJ, Fleischmann R, Deodhar AA, Wollenhaupt J, Khraishi M, Kielar D, Woltering F, Stach C, Hoepken B, Arledge T, van der Heijde D (January 2014). "Effect of certolizumab pegol on signs and symptoms in patients with psoriatic arthritis: 24-week results of a Phase 3 double-blind randomised placebo-controlled study (RAPID-PsA)". Ann. Rheum. Dis. 73 (1): 48–55. doi:10.1136/annrheumdis-2013-203696. PMC 3888622. PMID 23942868.
- ↑ McInnes IB, Mease PJ, Kirkham B, Kavanaugh A, Ritchlin CT, Rahman P, van der Heijde D, Landewé R, Conaghan PG, Gottlieb AB, Richards H, Pricop L, Ligozio G, Patekar M, Mpofu S (September 2015). "Secukinumab, a human anti-interleukin-17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double-blind, placebo-controlled, phase 3 trial". Lancet. 386 (9999): 1137–46. doi:10.1016/S0140-6736(15)61134-5. PMID 26135703.
- ↑ Mease PJ, van der Heijde D, Ritchlin CT, Okada M, Cuchacovich RS, Shuler CL, Lin CY, Braun DK, Lee CH, Gladman DD (January 2017). "Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1". Ann. Rheum. Dis. 76 (1): 79–87. doi:10.1136/annrheumdis-2016-209709. PMC 5264219. PMID 27553214.
- ↑ McInnes IB, Kavanaugh A, Gottlieb AB, Puig L, Rahman P, Ritchlin C, Brodmerkel C, Li S, Wang Y, Mendelsohn AM, Doyle MK (August 2013). "Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double-blind, placebo-controlled PSUMMIT 1 trial". Lancet. 382 (9894): 780–9. doi:10.1016/S0140-6736(13)60594-2. PMID 23769296.
- ↑ Mease PJ, Gottlieb AB, van der Heijde D, FitzGerald O, Johnsen A, Nys M, Banerjee S, Gladman DD (September 2017). "Efficacy and safety of abatacept, a T-cell modulator, in a randomised, double-blind, placebo-controlled, phase III study in psoriatic arthritis". Ann. Rheum. Dis. 76 (9): 1550–1558. doi:10.1136/annrheumdis-2016-210724. PMC 5561378. PMID 28473423.
- ↑ Nash P, Lubrano E, Cauli A, Taylor WJ, Olivieri I, Gladman DD (November 2014). "Updated guidelines for the management of axial disease in psoriatic arthritis". J. Rheumatol. 41 (11): 2286–9. doi:10.3899/jrheum.140877. PMID 25362712.
- ↑ Mease PJ, Armstrong AW (March 2014). "Managing patients with psoriatic disease: the diagnosis and pharmacologic treatment of psoriatic arthritis in patients with psoriasis". Drugs. 74 (4): 423–41. doi:10.1007/s40265-014-0191-y. PMC 3958815. PMID 24566842.
- ↑ Crowley JJ, Weinberg JM, Wu JJ, Robertson AD, Van Voorhees AS (January 2015). "Treatment of nail psoriasis: best practice recommendations from the Medical Board of the National Psoriasis Foundation". JAMA Dermatol. 151 (1): 87–94. doi:10.1001/jamadermatol.2014.2983. PMID 25471223.
- ↑ Rose S, Toloza S, Bautista-Molano W, Helliwell PS (November 2014). "Comprehensive treatment of dactylitis in psoriatic arthritis". J. Rheumatol. 41 (11): 2295–300. doi:10.3899/jrheum.140879. PMID 25362714.
- ↑ Zangger P, Esufali ZH, Gladman DD, Bogoch ER (April 2000). "Type and outcome of reconstructive surgery for different patterns of psoriatic arthritis". J. Rheumatol. 27 (4): 967–74. PMID 10782824.