Prostaglandin: Difference between revisions
No edit summary |
Varun Kumar (talk | contribs) m (→Biosynthesis) |
||
| Line 20: | Line 20: | ||
[[Image:Eicosanoid_synthesis_svg.png|thumb|left|300px|Biosynthesis of eicosanoids. (series-2)]]Prostaglandins are found in virtually all tissues and organs. These are [[autocrine]] and [[paracrine]] lipid mediators that act upon [[platelet]], [[endothelium]], [[uterus|uterine]] and [[mast cell]]s, among others. They are synthesized in the cell from the [[essential fatty acid]]s<ref name="merck">Dorlands Medical Dictionary [http://www.mercksource.com/pp/us/cns/cns_hl_dorlands.jspzQzpgzEzzSzppdocszSzuszSzcommonzSzdorlandszSzdorlandzSzdmd_p_36zPzhtm#1085994] URL reference on 10/23/05.</ref> (EFAs). | [[Image:Eicosanoid_synthesis_svg.png|thumb|left|300px|Biosynthesis of eicosanoids. (series-2)]]Prostaglandins are found in virtually all tissues and organs. These are [[autocrine]] and [[paracrine]] lipid mediators that act upon [[platelet]], [[endothelium]], [[uterus|uterine]] and [[mast cell]]s, among others. They are synthesized in the cell from the [[essential fatty acid]]s<ref name="merck">Dorlands Medical Dictionary [http://www.mercksource.com/pp/us/cns/cns_hl_dorlands.jspzQzpgzEzzSzppdocszSzuszSzcommonzSzdorlandszSzdorlandzSzdmd_p_36zPzhtm#1085994] URL reference on 10/23/05.</ref> (EFAs). | ||
<br clear="left"/> | |||
{| class="wikitable" | {| class="wikitable" | ||
| '''Name''' || '''EFA Type''' || '''Series''' | | '''Name''' || '''EFA Type''' || '''Series''' | ||
Latest revision as of 20:33, 28 September 2011
|
WikiDoc Resources for Prostaglandin |
|
Articles |
|---|
|
Most recent articles on Prostaglandin Most cited articles on Prostaglandin |
|
Media |
|
Powerpoint slides on Prostaglandin |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Prostaglandin at Clinical Trials.gov Trial results on Prostaglandin Clinical Trials on Prostaglandin at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Prostaglandin NICE Guidance on Prostaglandin
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Prostaglandin Discussion groups on Prostaglandin Patient Handouts on Prostaglandin Directions to Hospitals Treating Prostaglandin Risk calculators and risk factors for Prostaglandin
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Prostaglandin |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [3]
Overview
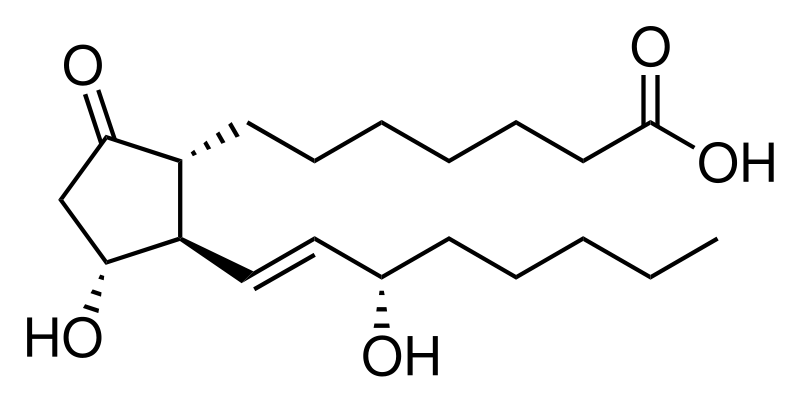
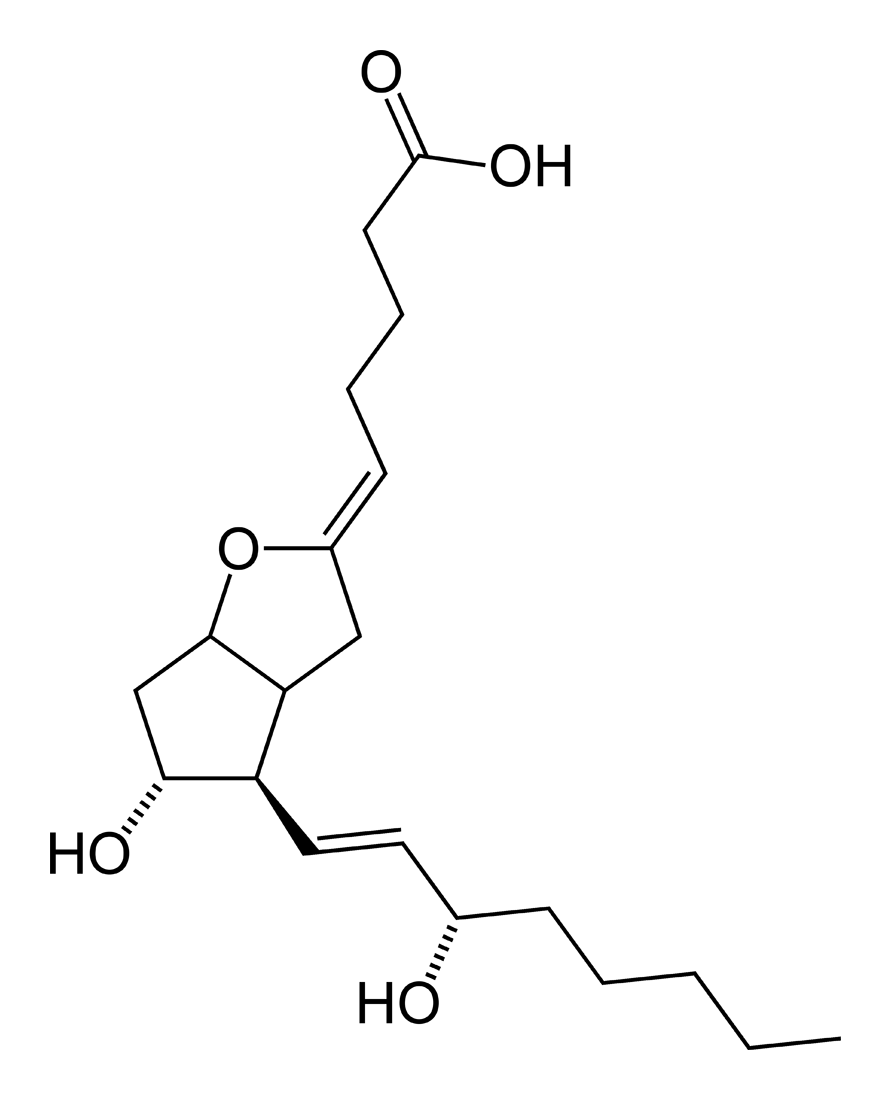
A prostaglandin is any member of a group of lipid compounds that are derived enzymatically from fatty acids and have important functions in the animal body. Every prostaglandin contains 20 carbon atoms, including a 5-carbon ring. They are mediators and have a variety of strong physiological effects; although they are technically hormones, they are rarely classified as such.
The prostaglandins together with the thromboxanes and prostacyclins form the prostanoid class of fatty acid derivatives; the prostanoid class is a subclass of eicosanoids.
History and name
The name prostaglandin derives from the prostate gland. When prostaglandin was first isolated from seminal fluid in 1935 by the Swedish physiologist Ulf von Euler,[1] and independently by M.W. Goldblatt,[2] it was believed to be part of the prostatic secretions (in actuality prostaglandins are produced by the seminal vesicles); it was later shown that many other tissues secrete prostaglandins for various functions.
In 1971, it was determined that aspirin-like drugs could inhibit the synthesis of prostaglandins. The biochemists Sune K. Bergström, Bengt I. Samuelsson and John R. Vane jointly received the 1982 Nobel Prize in Physiology or Medicine for their researches on prostaglandins.
Biochemistry
Biosynthesis
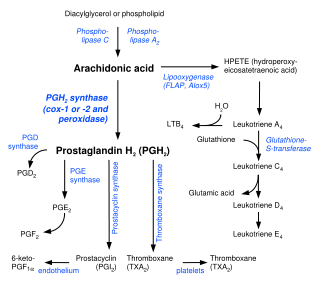
Prostaglandins are found in virtually all tissues and organs. These are autocrine and paracrine lipid mediators that act upon platelet, endothelium, uterine and mast cells, among others. They are synthesized in the cell from the essential fatty acids[3] (EFAs).
| Name | EFA Type | Series |
| Gamma-linolenic acid (GLA) via DGLA | ω-6 | series-1 |
| Arachidonic acid (AA) | ω-6 | series-2 |
| Eicosapentaenoic acid (EPA) | ω-3 | series-3 |
An intermediate is created by phospholipase-A2, then passed into one of either the cyclooxygenase pathway or the lipoxygenase pathway to form either prostaglandin and thromboxane or leukotriene. The cyclooxygenase pathway produces thromboxane, prostacyclin and prostaglandin D, E and F. The lipoxygenase pathway is active in leukocytes and in macrophages and synthesizes leukotrienes.
Release of prostaglandins from the cell
Prostaglandins were originally believed to leave the cells via passive diffusion because of their high lipophilicity. The discovery of the prostaglandin transporter (PGT, SLCO2A1), which mediates the cellular uptake of prostaglandin, demonstrated that diffusion can not explain the penetration of prostaglandin through the cellular membrane. The release of prostaglandin has now also been shown to be mediated by a specific transporter, namely the multidrug resistance protein 4 (MRP4, ABCC4), a member of the ATP-binding cassette transporter superfamily. Whether MRP4 is the only transporter releasing prostaglandins from the cells is still unclear.
Cyclooxygenases
Prostaglandins are produced following the sequential oxidation of AA, DGLA or EPA by cyclooxygenases (COX-1 and COX-2) and terminal prostaglandin synthases. The classic dogma is as follows:
- COX-1 is responsible for the baseline levels of prostaglandins.
- COX-2 produces prostaglandins through stimulation.
However, while COX-1 and COX-2 are both located in the blood vessels, stomach and the kidneys, prostaglandin levels are increased by COX-2 in scenarios of inflammation.
Prostaglandin E synthase
Prostaglandin E2 (PGE2) is generated from the action of prostaglandin E synthases on prostaglandin H2 (PGH2). Several prostaglandin E synthases have been identified. To date, microsomal prostaglandin E synthase-1 emerges as a key enzyme in the formation of PGE2.
Other terminal prostaglandin synthases
Terminal prostaglandin synthases have been identified that are responsible for the formation of other prostaglandins. For example, hematopoietic and lipocalin prostaglandin D synthases (hPGDS and lPGDS) are responsible for the formation of PGD2 from PGH2. Similarly, prostacyclin (PGI2) synthase (PGIS) converts PGH2 into PGI2. A thromboxane synthase (TxAS) has also been idenfitied. Prostaglandin F synthase (PGFS) catalyzes the formation of 9α,11β-PGF2α,β from PGD2 and PGF2α from PGH2 in the presence of NADPH. This enzyme has recently been crystallyzed in complex with PGD2[4] and bimatoprost[5] (a synthetic analogue of PGF2α).
Function
There are currently nine known prostaglandin receptors on various cell types. Prostaglandins ligate a subfamily of cell surface seven-transmembrane receptors, G-protein-coupled receptors. These receptors are termed DP1-2, EP1-4, FP, IP, and TP, corresponding to the receptor that ligates the corresponding prostaglandin (e.g., DP1-2 receptors bind to PGD2).
These varied receptors mean that Prostaglandins thus act on a variety of cells, and have a wide variety of actions:
- cause constriction or dilatation in vascular smooth muscle cells
- cause aggregation or disaggregation of platelets
- sensitize spinal neurons to pain
- constrict smooth muscle
- regulate inflammatory mediation
- regulate calcium movement
- regulate hormone regulation
- control cell growth
Prostaglandins are potent but have a short half-life before being inactivated and excreted. Therefore, they exert only a paracrine (locally active) or autocrine (acting on the same cell from which it is synthesized) function.
Role in pharmacology
Inhibition
Clinical uses
Synthetic prostaglandins are used:
- To induce childbirth, parturition or abortion (PGE2 or PGF2, with or without mifepristone, a progesterone antagonist);
- To prevent closure of patent ductus arteriosus in newborns with particular cyanotic heart defects (PGE1)
- To prevent and treat peptic ulcers (PGE)
- As a vasodilator in severe Raynaud's phenomenon or ischemia of a limb
- In pulmonary hypertension
- In treatment of glaucoma (as in bimatoprost ophthalmic solution, a synthetic prostamide analog with ocular hypotensive activity)
- To treat erectile dysfunction or in penile rehabilitation following surgery (PGE1 as alprostadil).[6]
References
- ↑ Von Euler US. Über die spezifische blutdrucksenkende Substanz des menschlichen Prostata- und Samenblasensekrets. Klin Wochenschr 1935;14:1182–1183.
- ↑ Goldblatt MW. Properties of human seminal plasma. J Physiol 1935;84:208-18. PMID 16994667.
- ↑ Dorlands Medical Dictionary [1] URL reference on 10/23/05.
- ↑ Komoto J, Yamada T, Watanabe K, Takusagawa F (2004). "Crystal structure of human prostaglandin F synthase (AKR1C3)". Biochemistry. 43 (8): 2188–98. PMID 14979715.
- ↑ Komoto J, Yamada T, Watanabe K, Woodward D, Takusagawa F (2006). "Prostaglandin F2alpha formation from prostaglandin H2 by prostaglandin F synthase (PGFS): crystal structure of PGFS containing bimatoprost". Biochemistry. 45 (7): 1987–96. PMID 16475787.
- ↑ Medscape Early Penile Rehabilitation Helps Reduce Later Intractable ED [2] URL reference on 10/23/05.
Template:Eicosanoids Template:Prostaglandins
de:Prostaglandin it:Prostaglandine lt:Prostaglandinai nl:Prostaglandinen no:Prostaglandin nn:Prostaglandin sl:Prostaglandin fi:Prostaglandiini sv:Prostaglandin uk:Простагландини