Procaine: Difference between revisions
m (Protected "Procaine": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
Gerald Chi (talk | contribs) m (Changed protection level for "Procaine" ([Edit=Allow only autoconfirmed users] (expires 12:06, 25 July 2014 (UTC)) [Move=Allow only autoconfirmed users] (expires 12:06, 25 July 2014 (UTC)))) |
(No difference)
| |
Revision as of 12:06, 25 June 2014
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
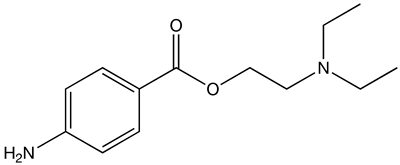 | |
| Clinical data | |
|---|---|
| Pregnancy category | |
| Routes of administration | Parenteral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Metabolism | Hydrolysis by plasma esterases |
| Elimination half-life | 40–84 seconds |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C13H20N2O2 |
| Molar mass | 236.31 g/mol |
|
WikiDoc Resources for Procaine |
|
Articles |
|---|
|
Most recent articles on Procaine |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Procaine at Clinical Trials.gov Clinical Trials on Procaine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Procaine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Directions to Hospitals Treating Procaine Risk calculators and risk factors for Procaine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Procaine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Procaine is a local anesthetic drug of the amino ester group. It is used primarily to reduce the pain of intramuscular injection of penicillin, and is also used in dentistry. Owing to the ubiquity of the trade name Novocain, procaine is sometimes referred to generically as novocaine or novacaine.
Procaine was first synthesized in 1905, and was the first injectable man-made local anesthetic used. It was created by the German chemist Alfred Einhorn (1857–1917) who gave the chemical the trade name Novocaine, from the Latin Novus (meaning New) and caine, a common ending for alkaloids used as anesthetics. It was introduced into medical use by surgeon Heinrich Braun (1862–1934).
Procaine is used less frequently today since more effective (and hypoallergenic) alternatives such as lidocaine (xylocaine) exist. Prior to the discovery of procaine, cocaine was the most commonly used local anesthetic. Procaine (like cocaine) has the advantage of constricting blood vessels, which reduces bleeding, unlike other local anesthetics like lidocaine, and without the euphoric and addictive qualities of cocaine.
Procaine, an ester anesthetic, is metabolized in the plasma by the enzyme pseudocholinesterase through hydrolysis into para-amino benzoic acid (PABA), which is then excreted by the kidneys into the urine. Allergic reactions to procaine are usually not in response to procaine itself, but to PABA. About 1 in 3000 people have an atypical form of pseudocholinesterase, which doesn't hydrolyze ester anesthetics such as procaine, resulting in a prolonged period of high levels of the anesthetic in the blood and increased toxicity.
Procaine is the primary ingredient in the controversial preparation Gerovital H3, which is claimed by its advocates to remedy many effects of aging. The mainstream medical view is that these claims were seriously studied and discredited in the 1960s.
See also
Template:SIB Template:Vasoprotectives
de:Procain it:Procaina nl:Novocaïne simple:Procaine sv:Prokain
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- Dental equipment
- Local anesthetics
- 1905 introductions