Procainamide: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
{{ | |authorTag= | ||
Gerald Chi | |||
<!--Overview--> | |||
|genericName= | |||
|aOrAn= | |||
a | |||
|drugClass= | |||
|indication= | |||
|hasBlackBoxWarning= | |||
Yes | |||
|adverseReactions= | |||
< | <!--Black Box Warning--> | ||
|blackBoxWarningTitle= | |||
Title | |||
| | |blackBoxWarningBody= | ||
</ | <i><span style="color:#FF0000;">ConditionName: </span></i> | ||
* Content | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
|fdaLIADAdult= | |||
=====Condition1===== | |||
{{ | * Dosing Information | ||
:* Dosage | |||
=====Condition2===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition3===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition4===== | |||
* Dosing Information | |||
:* Dosage | |||
<!--Off-Label Use and Dosage (Adult)--> | |||
<!--Guideline-Supported Use (Adult)--> | |||
|offLabelAdultGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | |||
|offLabelAdultNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Pediatric Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | |||
|fdaLIADPed= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>FDA-Labeled Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | |||
<!--Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedGuideSupport= | |||
=====Condition1===== | |||
* Developed by: | |||
* Class of Recommendation: | |||
* Strength of Evidence: | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport= | |||
=====Condition1===== | |||
* Dosing Information | |||
:* Dosage | |||
=====Condition2===== | |||
There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Contraindications--> | |||
|contraindications= | |||
* Condition1 | |||
<!--Warnings--> | |||
|warnings= | |||
* Description | |||
====Precautions==== | |||
* Description | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials= | |||
There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | |||
=====Body as a Whole===== | |||
=====Cardiovascular===== | |||
=====Digestive===== | |||
=====Endocrine===== | |||
=====Hematologic and Lymphatic===== | |||
=====Metabolic and Nutritional===== | |||
=====Musculoskeletal===== | |||
=====Neurologic===== | |||
=====Respiratory===== | |||
=====Skin and Hypersensitivy Reactions===== | |||
=====Special Senses===== | |||
=====Urogenital===== | |||
=====Miscellaneous===== | |||
<!--Postmarketing Experience--> | |||
|postmarketing= | |||
There is limited information regarding <i>Postmarketing Experience</i> of {{PAGENAME}} in the drug label. | |||
=====Body as a Whole===== | |||
=====Cardiovascular===== | |||
=====Digestive===== | |||
=====Endocrine===== | |||
=====Hematologic and Lymphatic===== | |||
=====Metabolic and Nutritional===== | |||
=====Musculoskeletal===== | |||
=====Neurologic===== | |||
=====Respiratory===== | |||
=====Skin and Hypersensitivy Reactions===== | |||
=====Special Senses===== | |||
=====Urogenital===== | |||
=====Miscellaneous===== | |||
<!--Drug Interactions--> | |||
|drugInteractions= | |||
* Drug | |||
:* Description | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA= | |||
* '''Pregnancy Category''' | |||
|useInPregnancyAUS= | |||
* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery= | |||
There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to nursing mothers. | |||
|useInPed= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to pediatric patients. | |||
|useInGeri= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to geriatric patients. | |||
|useInGender= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace= | |||
There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair= | |||
There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential= | |||
There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp= | |||
There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration= | |||
* Oral | |||
* Intravenous | |||
|monitoring= | |||
There is limited information regarding <i>Monitoring</i> of {{PAGENAME}} in the drug label. | |||
=====Condition1===== | |||
* Description | |||
<!--IV Compatibility--> | |||
|IVCompat= | |||
There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose= | |||
===Acute Overdose=== | |||
====Signs and Symptoms==== | |||
* Description | |||
====Management==== | |||
* Description | |||
===Chronic Overdose=== | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacology--> | |||
<!--Drug box 2--> | |||
|drugBox= | |||
<!--Mechanism of Action--> | |||
|mechAction= | |||
* | |||
<!--Structure--> | |||
|structure= | |||
* | |||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
<!--Pharmacodynamics--> | |||
|PD= | |||
There is limited information regarding <i>Pharmacodynamics</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacokinetics--> | |||
|PK= | |||
There is limited information regarding <i>Pharmacokinetics</i> of {{PAGENAME}} in the drug label. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic= | |||
There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
<!--Clinical Studies--> | |||
|clinicalStudies= | |||
There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
=====Condition1===== | |||
* Description | |||
<!--How Supplied--> | |||
|howSupplied= | |||
* | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo= | |||
There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
<!--Precautions with Alcohol--> | |||
|alcohol= | |||
* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames= | |||
* ®<ref>{{Cite web | title = | url = }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
|lookAlike= | |||
* A® — B®<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
<!--Drug Shortage Status--> | |||
|drugShortage= | |||
}} | |||
<!--Pill Image--> | |||
{{PillImage | |||
|fileName=No image.jpg|This image is provided by the National Library of Medicine. | |||
|drugName= | |||
|NDC= | |||
|drugAuthor= | |||
|ingredients= | |||
|pillImprint= | |||
|dosageValue= | |||
|dosageUnit= | |||
|pillColor= | |||
|pillShape= | |||
|pillSize= | |||
|pillScore= | |||
}} | |||
<!--Label Display Image--> | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}11.png|This image is provided by the National Library of Medicine. | |||
}} | |||
<!--Category--> | |||
[[Category:Cardiovascular Drugs]] | [[Category:Cardiovascular Drugs]] | ||
[[Category: | [[Category:Drug]] | ||
Revision as of 02:52, 13 July 2014
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
Overview
Procainamide is a that is FDA approved for the {{{indicationType}}} of . There is a Black Box Warning for this drug as shown here. Common adverse reactions include .
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Condition1
- Dosing Information
- Dosage
Condition2
- Dosing Information
- Dosage
Condition3
- Dosing Information
- Dosage
Condition4
- Dosing Information
- Dosage
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Procainamide in adult patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Procainamide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding FDA-Labeled Use of Procainamide in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
Condition1
- Developed by:
- Class of Recommendation:
- Strength of Evidence:
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Guideline-Supported Use of Procainamide in pediatric patients.
Non–Guideline-Supported Use
Condition1
- Dosing Information
- Dosage
Condition2
There is limited information regarding Off-Label Non–Guideline-Supported Use of Procainamide in pediatric patients.
Contraindications
- Condition1
Warnings
|
Title
See full prescribing information for complete Boxed Warning.
ConditionName:
|
- Description
Precautions
- Description
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Procainamide in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Procainamide in the drug label.
Body as a Whole
Cardiovascular
Digestive
Endocrine
Hematologic and Lymphatic
Metabolic and Nutritional
Musculoskeletal
Neurologic
Respiratory
Skin and Hypersensitivy Reactions
Special Senses
Urogenital
Miscellaneous
Drug Interactions
- Drug
- Description
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Procainamide in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Procainamide during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Procainamide with respect to nursing mothers.
Pediatric Use
There is no FDA guidance on the use of Procainamide with respect to pediatric patients.
Geriatic Use
There is no FDA guidance on the use of Procainamide with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Procainamide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Procainamide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Procainamide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Procainamide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Procainamide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Procainamide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
There is limited information regarding Monitoring of Procainamide in the drug label.
Condition1
- Description
IV Compatibility
There is limited information regarding IV Compatibility of Procainamide in the drug label.
Overdosage
Acute Overdose
Signs and Symptoms
- Description
Management
- Description
Chronic Overdose
There is limited information regarding Chronic Overdose of Procainamide in the drug label.
Pharmacology
There is limited information regarding Procainamide Pharmacology in the drug label.
Mechanism of Action
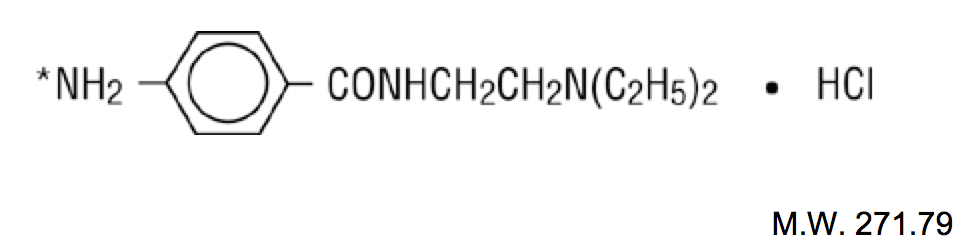
Structure
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Procainamide in the drug label.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Procainamide in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Procainamide in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Procainamide in the drug label.
Condition1
- Description
How Supplied
Storage
There is limited information regarding Procainamide Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Procainamide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Procainamide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Procainamide in the drug label.
Precautions with Alcohol
- Alcohol-Procainamide interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®[1]
Look-Alike Drug Names
- A® — B®[2]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Empty citation (help)
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Procainamide |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Procainamide |Label Name=Procainamide11.png
}}
{{#subobject:
|Label Page=Procainamide |Label Name=Procainamide11.png
}}